Oligodendrocyte-lineage cell exocytosis and L-type prostaglandin D synthase promote oligodendrocyte development and myelination
- PMID: 36779701
- PMCID: PMC9946447
- DOI: 10.7554/eLife.77441
Oligodendrocyte-lineage cell exocytosis and L-type prostaglandin D synthase promote oligodendrocyte development and myelination
Abstract
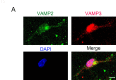
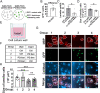
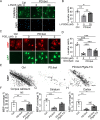
In the developing central nervous system, oligodendrocyte precursor cells (OPCs) differentiate into oligodendrocytes, which form myelin around axons. Oligodendrocytes and myelin are essential for the function of the central nervous system, as evidenced by the severe neurological symptoms that arise in demyelinating diseases such as multiple sclerosis and leukodystrophy. Although many cell-intrinsic mechanisms that regulate oligodendrocyte development and myelination have been reported, it remains unclear whether interactions among oligodendrocyte-lineage cells (OPCs and oligodendrocytes) affect oligodendrocyte development and myelination. Here, we show that blocking vesicle-associated membrane protein (VAMP) 1/2/3-dependent exocytosis from oligodendrocyte-lineage cells impairs oligodendrocyte development, myelination, and motor behavior in mice. Adding oligodendrocyte-lineage cell-secreted molecules to secretion-deficient OPC cultures partially restores the morphological maturation of oligodendrocytes. Moreover, we identified L-type prostaglandin D synthase as an oligodendrocyte-lineage cell-secreted protein that promotes oligodendrocyte development and myelination in vivo. These findings reveal a novel autocrine/paracrine loop model for the regulation of oligodendrocyte and myelin development.
Keywords: L-type prostaglandin D synthase; development; developmental biology; exocytosis; mouse; myelin; neuroscience; oligodendrocyte precursor cells; oligodendrocytes.
© 2023, Pan et al.
Conflict of interest statement
LP, AT, AZ, KF, YU, LS, CT No competing interests declared, YZ consulted for Ono Pharmaceutical
Figures

















References
-
- Aggarwal S, Snaidero N, Pähler G, Frey S, Sánchez P, Zweckstetter M, Janshoff A, Schneider A, Weil MT, Schaap IAT, Görlich D, Simons M. Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork. PLOS Biology. 2013;11:e1001577. doi: 10.1371/journal.pbio.1001577. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

