Continuous sensing of IFNα by hepatic endothelial cells shapes a vascular antimetastatic barrier
- PMID: 36281643
- PMCID: PMC9596162
- DOI: 10.7554/eLife.80690
Continuous sensing of IFNα by hepatic endothelial cells shapes a vascular antimetastatic barrier
Abstract
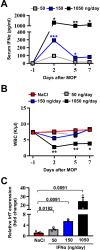
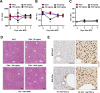
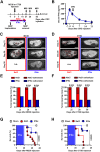
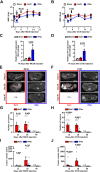
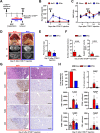
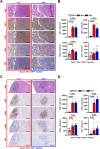
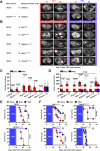
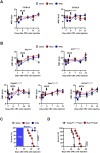
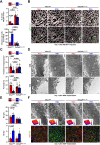
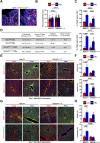
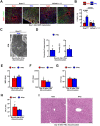
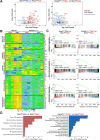
Hepatic metastases are a poor prognostic factor of colorectal carcinoma (CRC) and new strategies to reduce the risk of liver CRC colonization are highly needed. Herein, we used mouse models of hepatic metastatization to demonstrate that the continuous infusion of therapeutic doses of interferon-alpha (IFNα) controls CRC invasion by acting on hepatic endothelial cells (HECs). Mechanistically, IFNα promoted the development of a vascular antimetastatic niche characterized by liver sinusoidal endothelial cells (LSECs) defenestration extracellular matrix and glycocalyx deposition, thus strengthening the liver vascular barrier impairing CRC trans-sinusoidal migration, without requiring a direct action on tumor cells, hepatic stellate cells, hepatocytes, or liver dendritic cells (DCs), Kupffer cells (KCs) and liver capsular macrophages (LCMs). Moreover, IFNα endowed LSECs with efficient cross-priming potential that, along with the early intravascular tumor burden reduction, supported the generation of antitumor CD8+ T cells and ultimately led to the establishment of a protective long-term memory T cell response. These findings provide a rationale for the use of continuous IFNα therapy in perioperative settings to reduce CRC metastatic spreading to the liver.
Keywords: HECs; LSECs; cancer biology; colorectal cancer; cross-priming; immunology; inflammation; interferon-alpha; liver metastases; mouse.
Plain language summary
Colorectal cancer remains one of the most widespread and deadly cancers worldwide. Poor health outcomes are usually linked to diseased cells spreading from the intestine to create new tumors in the liver or other parts of the body. Treatment involves surgically removing the initial tumors in the bowel, but patient survival could be improved if, in parallel, their immune system was ‘boosted’ to destroy cancer cells before they can form other tumors. Interferon alpha is a small protein which helps to coordinate how the immune system recognizes and deactivates foreign agents and cancerous cells. It has recently been trialed as a colorectal cancer treatment to prevent tumors from spreading to the liver, but only with limited success. This partly because interferon-alpha is usually administered in high and pulsed doses, which cause severe side effects through the body. Instead, Tran, Ferreira, Alvarez-Moya et al. aimed to investigate whether continuously delivering lower amounts of the drug could be a better approach. This strategy was tested on mice in which colorectal cancer cells had been implanted into the wall of the large intestine. Continuous administration minimized the risk of the implanted cancer cells spreading to the liver while also creating fewer side effects. The team was able to identify an optimum delivery strategy by varying how much interferon-alpha the animals received and when. Further experiments also revealed a new mechanism by which interferon-alpha prevented the spread of colorectal cancer. Upon receiving continuous doses of the drug, a group of liver cells started to generate a physical barrier which stopped cancer cells from being able to invade the organ. The treatment also promoted long-term immune responses that targeted diseased cells while being safe for healthy tissues. If confirmed in clinical trials, these results suggest that colorectal patients undergoing tumor removal surgery may benefit from also receiving interferon-alpha through continuous delivery.
© 2022, Tran, Ferreira, Alvarez-Moya et al.
Conflict of interest statement
NT, LF, BA, VB, BF, PZ, AM, CF, EB, GS, AE, RL, CG, AB, GS No competing interests declared, LG LGG is a member of the board of directors at Genenta Science and Epsilon Bio and participates in advisory boards/consultancies for Gilead Sciences, Roche, Arbutus Biopharma and Antios Therapeutics
Figures



References
-
- Ananth AA, Tai L-H, Lansdell C, Alkayyal AA, Baxter KE, Angka L, Zhang J, Tanese de Souza C, Stephenson KB, Parato K, Bramson JL, Bell JC, Lichty BD, Auer RC. Surgical stress abrogates pre-existing protective T cell mediated anti-tumor immunity leading to postoperative cancer recurrence. PLOS ONE. 2016;11:e0155947. doi: 10.1371/journal.pone.0155947. - DOI - PMC - PubMed
-
- Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2020;31:1291–1305. doi: 10.1016/j.annonc.2020.06.022. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

