RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma
- PMID: 21289283
- PMCID: PMC3044382
- DOI: 10.1073/pnas.1012351108
RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma
Abstract
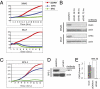
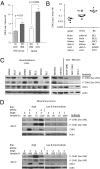
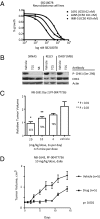
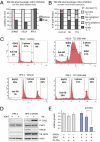
Neuroblastoma is a childhood cancer that is often fatal despite intense multimodality therapy. In an effort to identify therapeutic targets for this disease, we performed a comprehensive loss-of-function screen of the protein kinome. Thirty kinases showed significant cellular cytotoxicity when depleted, with loss of the cell cycle checkpoint kinase 1 (CHK1/CHEK1) being the most potent. CHK1 mRNA expression was higher in MYC-Neuroblastoma-related (MYCN)-amplified (P < 0.0001) and high-risk (P = 0.03) tumors. Western blotting revealed that CHK1 was constitutively phosphorylated at the ataxia telangiectasia response kinase target site Ser345 and the autophosphorylation site Ser296 in neuroblastoma cell lines. This pattern was also seen in six of eight high-risk primary tumors but not in control nonneuroblastoma cell lines or in seven of eight low-risk primary tumors. Neuroblastoma cells were sensitive to the two CHK1 inhibitors SB21807 and TCS2312, with median IC(50) values of 564 nM and 548 nM, respectively. In contrast, the control lines had high micromolar IC(50) values, indicating a strong correlation between CHK1 phosphorylation and CHK1 inhibitor sensitivity (P = 0.0004). Furthermore, cell cycle analysis revealed that CHK1 inhibition in neuroblastoma cells caused apoptosis during S-phase, consistent with its role in replication fork progression. CHK1 inhibitor sensitivity correlated with total MYC(N) protein levels, and inducing MYCN in retinal pigmented epithelial cells resulted in CHK1 phosphorylation, which caused growth inhibition when inhibited. These data show the power of a functional RNAi screen to identify tractable therapeutical targets in neuroblastoma and support CHK1 inhibition strategies in this disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Matthay KK, et al. Role of myeloablative therapy in improved outcome for high risk neuroblastoma: Review of recent Children's Cancer Group results. Eur J Cancer. 1995;31A:572–575. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

