Nr2f1a maintains atrial nkx2.5 expression to repress pacemaker identity within venous atrial cardiomyocytes of zebrafish
- PMID: 37184369
- PMCID: PMC10185342
- DOI: 10.7554/eLife.77408
Nr2f1a maintains atrial nkx2.5 expression to repress pacemaker identity within venous atrial cardiomyocytes of zebrafish
Abstract
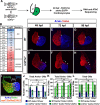
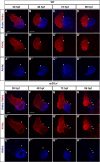
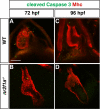
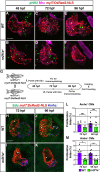
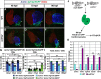
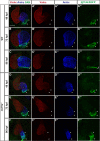
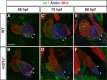
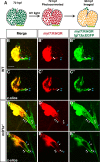
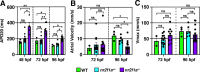
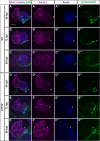
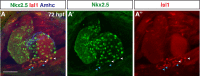
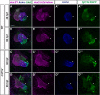
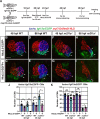
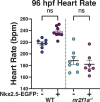
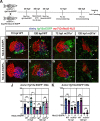
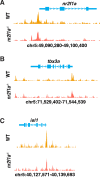
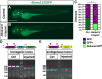
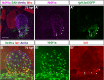
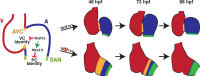
Maintenance of cardiomyocyte identity is vital for normal heart development and function. However, our understanding of cardiomyocyte plasticity remains incomplete. Here, we show that sustained expression of the zebrafish transcription factor Nr2f1a prevents the progressive acquisition of ventricular cardiomyocyte (VC) and pacemaker cardiomyocyte (PC) identities within distinct regions of the atrium. Transcriptomic analysis of flow-sorted atrial cardiomyocytes (ACs) from nr2f1a mutant zebrafish embryos showed increased VC marker gene expression and altered expression of core PC regulatory genes, including decreased expression of nkx2.5, a critical repressor of PC differentiation. At the arterial (outflow) pole of the atrium in nr2f1a mutants, cardiomyocytes resolve to VC identity within the expanded atrioventricular canal. However, at the venous (inflow) pole of the atrium, there is a progressive wave of AC transdifferentiation into PCs across the atrium toward the arterial pole. Restoring Nkx2.5 is sufficient to repress PC marker identity in nr2f1a mutant atria and analysis of chromatin accessibility identified an Nr2f1a-dependent nkx2.5 enhancer expressed in the atrial myocardium directly adjacent to PCs. CRISPR/Cas9-mediated deletion of the putative nkx2.5 enhancer leads to a loss of Nkx2.5-expressing ACs and expansion of a PC reporter, supporting that Nr2f1a limits PC differentiation within venous ACs via maintaining nkx2.5 expression. The Nr2f-dependent maintenance of AC identity within discrete atrial compartments may provide insights into the molecular etiology of concurrent structural congenital heart defects and associated arrhythmias.
Keywords: Nkx2.5 transcription factor; Nr2f transcription factor; atrial cardiomyocytes; cell biology; cellular plasticity; developmental biology; heart development; pacemaker cardiomyocyte; zebrafish.
© 2023, Martin et al.
Conflict of interest statement
KM, PR, MB, CM, JW No competing interests declared
Figures



Update of
- doi: 10.1101/2022.02.24.481762
References
-
- Al Turki S, Manickaraj AK, Mercer CL, Gerety SS, Hitz MP, Lindsay S, D’Alessandro LCA, Swaminathan GJ, Bentham J, Arndt AK, Louw J, Low J, Breckpot J, Gewillig M, Thienpont B, Abdul-Khaliq H, Harnack C, Hoff K, Kramer HH, Schubert S, Siebert R, Toka O, Cosgrove C, Watkins H, Lucassen AM, O’Kelly IM, Salmon AP, Bu’lock FA, Granados-Riveron J, Setchfield K, Thornborough C, Brook JD, Mulder B, Klaassen S, Bhattacharya S, Devriendt K, Fitzpatrick DF, Wilson DI, Mital S, Hurles ME, UK10K Consortium Rare variants in NR2F2 cause congenital heart defects in humans. American Journal of Human Genetics. 2014;94:574–585. doi: 10.1016/j.ajhg.2014.03.007. - DOI - PMC - PubMed
-
- Andrews S. FastQC: A quality control tool for high throughput sequence data. 2010. [March 19, 2019]. http://www.bioinformatics.babraham.ac.uk/projects/fastqc
-
- Barth AS, Merk S, Arnoldi E, Zwermann L, Kloos P, Gebauer M, Steinmeyer K, Bleich M, Kääb S, Pfeufer A, Uberfuhr P, Dugas M, Steinbeck G, Nabauer M. Functional profiling of human atrial and ventricular gene expression. Pflugers Archiv. 2005;450:201–208. doi: 10.1007/s00424-005-1404-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

