Transcriptional changes in Huntington disease identified using genome-wide expression profiling and cross-platform analysis
- PMID: 20089533
- PMCID: PMC2846159
- DOI: 10.1093/hmg/ddq018
Transcriptional changes in Huntington disease identified using genome-wide expression profiling and cross-platform analysis
Abstract
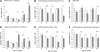
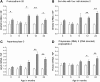
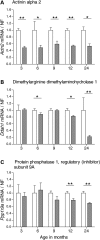
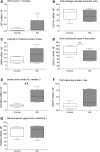
Evaluation of transcriptional changes in the striatum may be an effective approach to understanding the natural history of changes in expression contributing to the pathogenesis of Huntington disease (HD). We have performed genome-wide expression profiling of the YAC128 transgenic mouse model of HD at 12 and 24 months of age using two platforms in parallel: Affymetrix and Illumina. The data from these two powerful platforms were integrated to create a combined rank list, thereby revealing the identity of additional genes that proved to be differentially expressed between YAC128 and control mice. Using this approach, we identified 13 genes to be differentially expressed between YAC128 and controls which were validated by quantitative real-time PCR in independent cohorts of animals. In addition, we analyzed additional time points relevant to disease pathology: 3, 6 and 9 months of age. Here we present data showing the evolution of changes in the expression of selected genes: Wt1, Pcdh20 and Actn2 RNA levels change as early as 3 months of age, whereas Gsg1l, Sfmbt2, Acy3, Polr2a and Ppp1r9a RNA expression levels are affected later, at 12 and 24 months of age. We also analyzed the expression of these 13 genes in human HD and control brain, thereby revealing changes in SLC45A3, PCDH20, ACTN2, DDAH1 and PPP1R9A RNA expression. Further study of these genes may unravel novel pathways contributing to HD pathogenesis. DDBJ/EMBL/GenBank accession no: GSE19677.
Figures

References
-
- Gusella J.F. Group H.D.C. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. [see comment] Cell. 1993;72:971–983. - PubMed
-
- Furtado S., Suchowersky O., Rewcastle B., Graham L., Klimek M.L., Garber A. Relationship between trinucleotide repeats and neuropathological changes in Huntington's disease. Ann. Neurol. 1996;39:132–136. - PubMed
-
- Sotrel A., Paskevich P.A., Kiely D.K., Bird E.D., Williams R.S., Myers R.H. Morphometric analysis of the prefrontal cortex in Huntington's disease. Neurology. 1991;41:1117–1123. - PubMed
-
- Gil J.M., Rego A.C. Mechanisms of neurodegeneration in Huntington's disease. Eur. J. Neurosci. 2008;27:2803–2820. - PubMed
-
- Imarisio S., Carmichael J., Korolchuk V., Chen C.W., Saiki S., Rose C., Krishna G., Davies J.E., Ttofi E., Underwood B.R., et al. Huntington's disease: from pathology and genetics to potential therapies. Biochem. J. 2008;412:191–209. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

