Transcriptional profiling of Hutchinson-Gilford Progeria syndrome fibroblasts reveals deficits in mesenchymal stem cell commitment to differentiation related to early events in endochondral ossification
- PMID: 36579892
- PMCID: PMC9833827
- DOI: 10.7554/eLife.81290
Transcriptional profiling of Hutchinson-Gilford Progeria syndrome fibroblasts reveals deficits in mesenchymal stem cell commitment to differentiation related to early events in endochondral ossification
Abstract
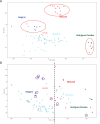
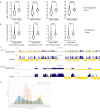
The expression of a mutant Lamin A, progerin, in Hutchinson-Gilford Progeria Syndrome leads to alterations in genome architecture, nuclear morphology, epigenetic states, and altered phenotypes in all cells of the mesenchymal lineage. Here, we report a comprehensive analysis of the transcriptional status of patient derived HGPS fibroblasts, including nine cell lines not previously reported, in comparison with age-matched controls, adults, and old adults. We find that Progeria fibroblasts carry abnormal transcriptional signatures, centering around several functional hubs: DNA maintenance and epigenetics, bone development and homeostasis, blood vessel maturation and development, fat deposition and lipid management, and processes related to muscle growth. Stratification of patients by age revealed misregulated expression of genes related to endochondral ossification and chondrogenic commitment in children aged 4-7 years old, where this differentiation program starts in earnest. Hi-C measurements on patient fibroblasts show weakening of genome compartmentalization strength but increases in TAD strength. While the majority of gene misregulation occurs in regions which do not change spatial chromosome organization, some expression changes in key mesenchymal lineage genes coincide with lamin associated domain misregulation and shifts in genome compartmentalization.
Keywords: 3D genome folding; chromosomes; developmental biology; gene expression; human; premature aging; transcriptomics.
© 2022, San Martin et al.
Conflict of interest statement
RS, PD, JS, AH, RM No competing interests declared
Figures












References
-
- Aguado J, Sola-Carvajal A, Cancila V, Revêchon G, Ong PF, Jones-Weinert CW, Wallén Arzt E, Lattanzi G, Dreesen O, Tripodo C, Rossiello F, Eriksson M, d’Adda di Fagagna F. Inhibition of DNA damage response at telomeres improves the detrimental phenotypes of hutchinson-gilford progeria syndrome. Nature Communications. 2019;10:4990. doi: 10.1038/s41467-019-13018-3. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

