NLR family member NLRC5 is a transcriptional regulator of MHC class I genes
- PMID: 20639463
- PMCID: PMC2922274
- DOI: 10.1073/pnas.1008684107
NLR family member NLRC5 is a transcriptional regulator of MHC class I genes
Abstract
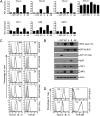
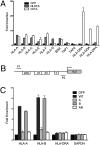
MHC class I plays a critical role in the immune defense against viruses and tumors by presenting antigens to CD8 T cells. An NLR protein, class II transactivator (CIITA), is a key regulator of MHC class II gene expression that associates and cooperates with transcription factors in the MHC class II promoter. Although CIITA also transactivates MHC class I gene promoters, loss of CIITA in humans and mice results in the severe reduction of only MHC class II expression, suggesting that additional mechanisms regulate the expression of MHC class I. Here, we identify another member of the NLR protein family, NLRC5, as a transcriptional regulator of MHC class I genes. Similar to CIITA, NLRC5 is an IFN-gamma-inducible nuclear protein, and the expression of NLRC5 resulted in enhanced MHC class I expression in lymphoid as well as epithelial cell lines. Using chromatin immunoprecipitation and reporter gene assays, we show that NLRC5 associates with and activates the promoters of MHC class I genes. Furthermore, we show that the IFN-gamma-induced up-regulation of MHC class I requires NLRC5, because knockdown of NLRC5 specifically impaired the expression of MHC class I. In addition to MHC class I genes, NLRC5 also induced the expression of beta2-microglobulin, transporter associated with antigen processing, and large multifunctional protease, which are essential for MHC class I antigen presentation. Our results suggest that NLRC5 is a transcriptional regulator, orchestrating the concerted expression of critical components in the MHC class I pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pamer E, Cresswell P. Mechanisms of MHC class I—restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. - PubMed
-
- Braud VM, Allan DS, McMichael AJ. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr Opin Immunol. 1999;11:100–108. - PubMed
-
- Le Bouteiller P, Solier C. Is antigen presentation the primary function of HLA-G? Microbes Infect. 2001;3:323–332. - PubMed
-
- Shastri N, Cardinaud S, Schwab SR, Serwold T, Kunisawa J. All the peptides that fit: The beginning, the middle, and the end of the MHC class I antigen-processing pathway. Immunol Rev. 2005;207:31–41. - PubMed
-
- Peaper DR, Cresswell P. Regulation of MHC class I assembly and peptide binding. Annu Rev Cell Dev Biol. 2008;24:343–368. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

