Apoptosis recognition receptors regulate skin tissue repair in mice
- PMID: 38127424
- PMCID: PMC10735221
- DOI: 10.7554/eLife.86269
Apoptosis recognition receptors regulate skin tissue repair in mice
Abstract
Apoptosis and clearance of apoptotic cells via efferocytosis are evolutionarily conserved processes that drive tissue repair. However, the mechanisms by which recognition and clearance of apoptotic cells regulate repair are not fully understood. Here, we use single-cell RNA sequencing to provide a map of the cellular dynamics during early inflammation in mouse skin wounds. We find that apoptotic pathways and efferocytosis receptors are elevated in fibroblasts and immune cells, including resident Lyve1+ macrophages, during inflammation. Interestingly, human diabetic foot wounds upregulate mRNAs for efferocytosis pathway genes and display altered efferocytosis signaling via the receptor Axl and its ligand Gas6. During early inflammation in mouse wounds, we detect upregulation of Axl in dendritic cells and fibroblasts via TLR3-independent mechanisms. Inhibition studies in vivo in mice reveal that Axl signaling is required for wound repair but is dispensable for efferocytosis. By contrast, inhibition of another efferocytosis receptor, Timd4, in mouse wounds decreases efferocytosis and abrogates wound repair. These data highlight the distinct mechanisms by which apoptotic cell detection coordinates tissue repair and provides potential therapeutic targets for chronic wounds in diabetic patients.
Keywords: apoptosis; cell biology; efferocytosis; human; immunology; inflammation; mouse; repair; skin; wound healing.
Plain language summary
Our skin is constantly exposed to potential damage from the outside world, and it is vital that any injuries are repaired quickly and effectively. Diabetes and many other health conditions can hamper wound healing, resulting in chronic wounds that are both painful and at risk of becoming infected, which can lead to serious illness and death of patients. After an injury to the skin, the wound becomes inflamed as immune cells rush to the site of injury to fight off infection and clear the wound of dead cells and debris. Some of these dead cells will have died by a highly controlled process known as apoptosis. These so-called apoptotic cells display signals on their surface that nearby healthy cells recognize. This triggers the healthy cells to eat the apoptotic cells to remove them from the wound. Previous studies have linked changes in cell death and the removal of dead cells to chronic wounds in patients with diabetes, but it remains unclear how removing dead cells from the wound affects healing. Justynski et al. used a genetic technique called single-cell RNA sequencing to study the patterns of gene activity in mouse skin cells shortly after a wound. The experiments found that, as the area around the wound started to become inflamed, the wounded cells produced signals of apoptosis that in turn triggered nearby healthy cells to remove them. Other signals relating to the removal of dead cells were also widespread in the mouse wounds and treating the wounds with drugs that inhibit these signals resulted in multiple defects in the healing process. Further experiments used the same approach to study samples of tissue taken from foot wounds in human patients with or without diabetes. This revealed that several genes involved in the removal of dead cells were more highly expressed in the wounds of diabetic patients than in the wounds of other individuals. These findings indicate that for wounds to heal properly it is crucial for the body to detect and clear apoptotic cells from the wound site. Further studies building on this work may help to explain why some diabetic patients suffer from chronic wounds and help to develop more effective treatments for them.
© 2023, Justynski et al.
Conflict of interest statement
OJ, KB, WK, MF, QP, TS, KC, DK, HH, MG, SV, RD, KM No competing interests declared, VH Reviewing editor, eLife
Figures


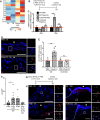
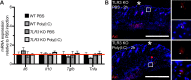


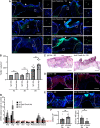
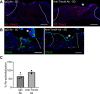
Update of
-
Apoptosis recognition receptors regulate skin tissue repair in mice.bioRxiv [Preprint]. 2023 Jan 17:2023.01.17.523241. doi: 10.1101/2023.01.17.523241. bioRxiv. 2023. Update in: Elife. 2023 Dec 21;12:e86269. doi: 10.7554/eLife.86269. PMID: 36711968 Free PMC article. Updated. Preprint.
References
-
- Bauer T, Zagórska A, Jurkin J, Yasmin N, Köffel R, Richter S, Gesslbauer B, Lemke G, Strobl H. Identification of Axl as a downstream effector of TGF-β1 during Langerhans cell differentiation and epidermal homeostasis. The Journal of Experimental Medicine. 2012;209:2033–2047. doi: 10.1084/jem.20120493. - DOI - PMC - PubMed
-
- Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, Gagliani N, Craft JE, Flavell RA, Ghosh S, Rothlin CV. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017a;356:1072–1076. doi: 10.1126/science.aai8132. - DOI - PMC - PubMed
MeSH terms
Associated data
- Actions
- Actions
Grants and funding
- R01 GM123011/GM/NIGMS NIH HHS/United States
- T32 GM007223/GM/NIGMS NIH HHS/United States
- R01 AR075412/AR/NIAMS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- K12 GM106996/GM/NIGMS NIH HHS/United States
- R01 AR079232/AR/NIAMS NIH HHS/United States
- T32 T32GM007223/NH/NIH HHS/United States
- R01-GM123011/NH/NIH HHS/United States
- R01 AR076938/AR/NIAMS NIH HHS/United States
- R01-CA238728/NH/NIH HHS/United States
- Predoctoral Training Program in Cellular and Molecular Biology/NH/NIH HHS/United States
- U01-CA238728/NH/NIH HHS/United States
- U01 CA238728/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

