The dysadherin/MMP9 axis modifies the extracellular matrix to accelerate colorectal cancer progression
- PMID: 39613801
- PMCID: PMC11607440
- DOI: 10.1038/s41467-024-54920-9
The dysadherin/MMP9 axis modifies the extracellular matrix to accelerate colorectal cancer progression
Abstract
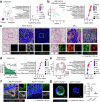
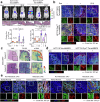
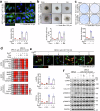
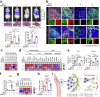
The dynamic alteration of the tumor microenvironment (TME) serves as a driving force behind the progression and metastasis of colorectal cancer (CRC). Within the intricate TME, a pivotal player is the extracellular matrix (ECM), where modifications in components, degradation, and stiffness are considered critical factors in tumor development. In this study, we find that the membrane glycoprotein dysadherin directly targets matrix metalloprotease 9 (MMP9), initiating ECM remodeling within the TME and amplifying cancer progression. Mechanistically, the dysadherin/MMP9 axis not only enhances CRC cell invasiveness and ECM proteolytic activity but also activates cancer-associated fibroblasts, orchestrating the restructuring of the ECM through the synthesis of its components in human CRC cells, patient samples, and mouse models. Notably, disruption of ECM reorganization by dysadherin knockout results in a discernible reduction in the immunosuppressive and proangiogenic milieu in a humanized mouse model. Intriguingly, these effects are reversed upon the overexpression of MMP9, highlighting the intricate and pivotal role of the dysadherin/MMP9 axis in shaping the development of a malignant TME. Therefore, our findings not only highlight that dysadherin contributes to CRC progression by influencing the TME through ECM remodeling but also suggest that dysadherin may be a potential therapeutic target for CRC.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Rahimi Pordanjani, S., Baeradeh, N., Lotfi, M. H. & Pourmohammadi, B. Epidemiology of colorectal cancer: incidence, mortality, survival rates and risk factors. Razi J. Med. Sci.23, 41–50 (2016).
-
- Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clinicians71, 209–249 (2021). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

