Protective effects of macrophage-specific integrin α5 in myocardial infarction are associated with accentuated angiogenesis
- PMID: 37985764
- PMCID: PMC10662477
- DOI: 10.1038/s41467-023-43369-x
Protective effects of macrophage-specific integrin α5 in myocardial infarction are associated with accentuated angiogenesis
Abstract
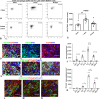
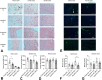
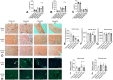
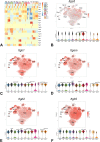
Macrophages sense changes in the extracellular matrix environment through the integrins and play a central role in regulation of the reparative response after myocardial infarction. Here we show that macrophage integrin α5 protects the infarcted heart from adverse remodeling and that the protective actions are associated with acquisition of an angiogenic macrophage phenotype. We demonstrate that myeloid cell- and macrophage-specific integrin α5 knockout mice have accentuated adverse post-infarction remodeling, accompanied by reduced angiogenesis in the infarct and border zone. Single cell RNA-sequencing identifies an angiogenic infarct macrophage population with high Itga5 expression. The angiogenic effects of integrin α5 in macrophages involve upregulation of Vascular Endothelial Growth Factor A. RNA-sequencing of the macrophage transcriptome in vivo and in vitro followed by bioinformatic analysis identifies several intracellular kinases as potential downstream targets of integrin α5. Neutralization assays demonstrate that the angiogenic actions of integrin α5-stimulated macrophages involve activation of Focal Adhesion Kinase and Phosphoinositide 3 Kinase cascades.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

