Canonical and alternate functions of the microRNA biogenesis machinery
- PMID: 20713509
- PMCID: PMC2932976
- DOI: 10.1101/gad.1953310
Canonical and alternate functions of the microRNA biogenesis machinery
Erratum in
- Genes Dev. 2010 Oct 1;24(19):2228
Abstract
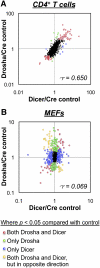
The canonical microRNA (miRNA) biogenesis pathway requires two RNaseIII enzymes: Drosha and Dicer. To understand their functions in mammals in vivo, we engineered mice with germline or tissue-specific inactivation of the genes encoding these two proteins. Changes in proteomic and transcriptional profiles that were shared in Dicer- and Drosha-deficient mice confirmed the requirement for both enzymes in canonical miRNA biogenesis. However, deficiency in Drosha or Dicer did not always result in identical phenotypes, suggesting additional functions. We found that, in early-stage thymocytes, Drosha recognizes and directly cleaves many protein-coding messenger RNAs (mRNAs) with secondary stem-loop structures. In addition, we identified a subset of miRNAs generated by a Dicer-dependent but Drosha-independent mechanism. These were distinct from previously described mirtrons. Thus, in mammalian cells, Dicer is required for the biogenesis of multiple classes of miRNAs. Together, these findings extend the range of function of RNaseIII enzymes beyond canonical miRNA biogenesis, and help explain the nonoverlapping phenotypes caused by Drosha and Dicer deficiency.
Figures




References
-
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ 2003. Dicer is essential for mouse development. Nat Genet 35: 215–217 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
