Protective effect of TCR-mediated MAIT cell activation during experimental autoimmune encephalomyelitis
- PMID: 39468055
- PMCID: PMC11519641
- DOI: 10.1038/s41467-024-53657-9
Protective effect of TCR-mediated MAIT cell activation during experimental autoimmune encephalomyelitis
Abstract
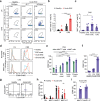
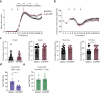
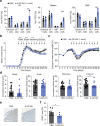
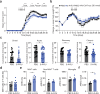
Mucosal-associated invariant T (MAIT) cells express semi-invariant T cell receptors (TCR) for recognizing bacterial and yeast antigens derived from riboflavin metabolites presented on the non-polymorphic MHC class I-related protein 1 (MR1). Neuroinflammation in multiple sclerosis (MS) is likely initiated by autoreactive T cells and perpetuated by infiltration of additional immune cells, but the precise role of MAIT cells in MS pathogenesis remains unknown. Here, we use experimental autoimmune encephalomyelitis (EAE), a mouse model of MS, and find an accumulation of MAIT cells in the inflamed central nervous system (CNS) enriched for MAIT17 (RORγt+) and MAIT1/17 (T-bet+RORγt+) subsets with inflammatory and protective features. Results from transcriptome profiling and Nur77GFP reporter mice show that these CNS MAIT cells are activated via cytokines and TCR. Blocking TCR activation with an anti-MR1 antibody exacerbates EAE, whereas enhancing TCR activation with the cognate antigen, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil, ameliorates EAE severity, potentially via the induction of amphiregulin (AREG). In summary, our findings suggest that TCR-mediated MAIT cell activation is protective in CNS inflammation, likely involving an induction of AREG.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Woo, M. S., Engler, J. B. & Friese, M. A. The neuropathology of multiple sclerosis. Nat. Rev. Neurosci.10.1038/s41583-024-00823-z (2024). - PubMed
-
- Attfield, K. E., Jensen, L. T., Kaufmann, M., Friese, M. A. & Fugger, L. The immunology of multiple sclerosis. Nat. Rev. Immunol.22, 734–750 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

