Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling
- PMID: 20962846
- PMCID: PMC3108066
- DOI: 10.1038/nature09447
Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling
Abstract
CD4(+) T-helper cells that selectively produce interleukin (IL)-17 (T(H)17), are critical for host defence and autoimmunity. Although crucial for T(H)17 cells in vivo, IL-23 has been thought to be incapable of driving initial differentiation. Rather, IL-6 and transforming growth factor (TGF)-β1 have been proposed to be the factors responsible for initiating specification. Here we show that T(H)17 differentiation can occur in the absence of TGF-β signalling. Neither IL-6 nor IL-23 alone efficiently generated T(H)17 cells; however, these cytokines in combination with IL-1β effectively induced IL-17 production in naive precursors, independently of TGF-β. Epigenetic modification of the Il17a, Il17f and Rorc promoters proceeded without TGF-β1, allowing the generation of cells that co-expressed RORγt (encoded by Rorc) and T-bet. T-bet(+)RORγt(+) T(H)17 cells are generated in vivo during experimental allergic encephalomyelitis, and adoptively transferred T(H)17 cells generated with IL-23 without TGF-β1 were pathogenic in this disease model. These data indicate an alternative mode for T(H)17 differentiation. Consistent with genetic data linking IL23R with autoimmunity, our findings re-emphasize the importance of IL-23 and therefore may have therapeutic implications.
Figures
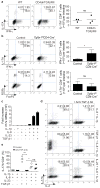
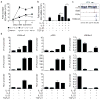

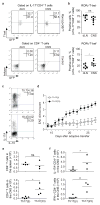
Comment in
-
Th17 cells generated in the absence of TGF-β induce experimental allergic encephalitis upon adoptive transfer.Expert Rev Clin Immunol. 2011 May;7(3):283-5. doi: 10.1586/eci.11.7. Expert Rev Clin Immunol. 2011. PMID: 21595594
References
-
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. - PubMed
-
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. - PubMed
-
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. - PubMed
-
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. - PubMed
-
- Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

