JAK inhibition decreases the autoimmune burden in Down syndrome
- PMID: 39737640
- PMCID: PMC11687933
- DOI: 10.7554/eLife.99323
JAK inhibition decreases the autoimmune burden in Down syndrome
Abstract
Background: Individuals with Down syndrome (DS), the genetic condition caused by trisomy 21 (T21), display clear signs of immune dysregulation, including high rates of autoimmunity and severe complications from infections. Although it is well established that T21 causes increased interferon responses and JAK/STAT signaling, elevated autoantibodies, global immune remodeling, and hypercytokinemia, the interplay between these processes, the clinical manifestations of DS, and potential therapeutic interventions remain ill defined.
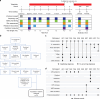
Methods: We report a comprehensive analysis of immune dysregulation at the clinical, cellular, and molecular level in hundreds of individuals with DS, including autoantibody profiling, cytokine analysis, and deep immune mapping. We also report the interim analysis of a Phase II clinical trial investigating the safety and efficacy of the JAK inhibitor tofacitinib through multiple clinical and molecular endpoints.
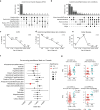
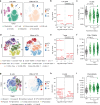
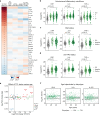
Results: We demonstrate multi-organ autoimmunity of pediatric onset concurrent with unexpected autoantibody-phenotype associations in DS. Importantly, constitutive immune remodeling and hypercytokinemia occur from an early age prior to autoimmune diagnoses or autoantibody production. Analysis of the first 10 participants to complete 16 weeks of tofacitinib treatment shows a good safety profile and no serious adverse events. Treatment reduced skin pathology in alopecia areata, psoriasis, and atopic dermatitis, while decreasing interferon scores, cytokine scores, and levels of pathogenic autoantibodies without overt immune suppression.
Conclusions: JAK inhibition is a valid strategy to treat autoimmune conditions in DS. Additional research is needed to define the effects of JAK inhibition on the broader developmental and clinical hallmarks of DS.
Funding: NIAMS, Global Down Syndrome Foundation.
Clinical trial number: NCT04246372.
Keywords: JAK; autoimmunity; down syndrome; human; immunology; inflammation; interferons; medicine; skin.
© 2024, Rachubinski et al.
Conflict of interest statement
AR, EW, EG, BE, KW, KS, PA, KW, RG, EB, HL, MD, NE, AH, BM, KS, LP, DF, MG, CD, DN No competing interests declared, JE has provided consulting services for Eli Lilly Co, Gilead Sciences Inc, and Biohaven Pharmaceuticals and serves on the advisory board of Perha Pharmaceuticals
Figures







Update of
-
JAK inhibition decreases the autoimmune burden in Down syndrome.medRxiv [Preprint]. 2024 Oct 16:2024.06.13.24308783. doi: 10.1101/2024.06.13.24308783. medRxiv. 2024. Update in: Elife. 2024 Dec 31;13:RP99323. doi: 10.7554/eLife.99323. PMID: 38946973 Free PMC article. Updated. Preprint.
References
-
- Aitken RJ, Mehers KL, Williams AJ, Brown J, Bingley PJ, Holl RW, Rohrer TR, Schober E, Abdul-Rasoul MM, Shield JPH, Gillespie KM. Early-onset, coexisting autoimmunity and decreased HLA-mediated susceptibility are the characteristics of diabetes in down syndrome. Diabetes Care. 2013;36:1181–1185. doi: 10.2337/dc12-1712. - DOI - PMC - PubMed
-
- Amagai M, Nishikawa T, Nousari HC, Anhalt GJ, Hashimoto T. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. The Journal of Clinical Investigation. 1998;102:775–782. doi: 10.1172/JCI3647. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- R01 AI150305/AI/NIAID NIH HHS/United States
- R61AR077495/AR/NIAMS NIH HHS/United States
- R01AI150305/National Institute of Allergy and Infectious Diseases
- P30 CA046934/CA/NCI NIH HHS/United States
- T32CA190216/CA/NCI NIH HHS/United States
- R61 AR077495/AR/NIAMS NIH HHS/United States
- UM1 TR004399/TR/NCATS NIH HHS/United States
- 2T32AR007411-31/AR/NIAMS NIH HHS/United States
- T32 AR007411/AR/NIAMS NIH HHS/United States
- T32 CA190216/CA/NCI NIH HHS/United States
- UM1TR004399/TR/NCATS NIH HHS/United States
- P30CA046934/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

