Diet modulates the therapeutic effects of dimethyl fumarate mediated by the immunometabolic neutrophil receptor HCAR2
- PMID: 40266880
- PMCID: PMC12113270
- DOI: 10.7554/eLife.98970
Diet modulates the therapeutic effects of dimethyl fumarate mediated by the immunometabolic neutrophil receptor HCAR2
Abstract
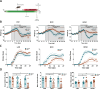
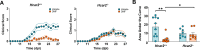
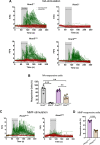
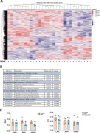
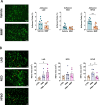
Monomethyl fumarate (MMF) and its prodrug dimethyl fumarate (DMF) are currently the most widely used agents for the treatment of multiple sclerosis (MS). However, not all patients benefit from DMF. We hypothesized that the variable response of patients may be due to their diet. In support of this hypothesis, mice subjected to experimental autoimmune encephalomyelitis (EAE), a model of MS, did not benefit from DMF treatment when fed a lauric acid (LA)-rich diet. Mice on normal chow (NC) diet, in contrast, and even more so mice on high-fiber (HFb) diet showed the expected protective DMF effect. DMF lacked efficacy in the LA diet-fed group despite similar resorption and preserved effects on plasma lipids. When mice were fed the permissive HFb diet, the protective effect of DMF treatment depended on hydroxycarboxylic receptor 2 (HCAR2), which is highly expressed in neutrophil granulocytes. Indeed, deletion of Hcar2 in neutrophils abrogated DMF protective effects in EAE. Diet had a profound effect on the transcriptional profile of neutrophils and modulated their response to MMF. In summary, DMF required HCAR2 on neutrophils as well as permissive dietary effects for its therapeutic action. Translating the dietary intervention into the clinic may improve MS therapy.
Keywords: experimental autoimmune encephalomyelitis; medicine; mouse; multiple sclerosis; neuroscience; neutrophils.
© 2025, Kosinska, Assmann et al.
Conflict of interest statement
JK, JA, JI, HM, KH, SG, AK, HB, AW, JH, CS, MG, VP, RN, MH, MS, SO, NW, MS No competing interests declared
Figures

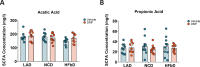







References
-
- Aherrahrou R, Kulle AE, Alenina N, Werner R, Vens-Cappell S, Bader M, Schunkert H, Erdmann J, Aherrahrou Z. CYP17A1 deficient XY mice display susceptibility to atherosclerosis, altered lipidomic profile and atypical sex development. Scientific Reports. 2020;10:8792. doi: 10.1038/s41598-020-65601-0. - DOI - PMC - PubMed
-
- Aitchison J. The Statistical Analysis of Compositional Data. London, UK: Chapman and Hall Ltd; 1986.
-
- Aubé B, Lévesque SA, Paré A, Chamma É, Kébir H, Gorina R, Lécuyer MA, Alvarez JI, De Koninck Y, Engelhardt B, Prat A, Côté D, Lacroix S. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. Journal of Immunology. 2014;193:2438–2454. doi: 10.4049/jimmunol.1400401. - DOI - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

