Cannabidiol ameliorates mitochondrial disease via PPARγ activation in preclinical models
- PMID: 39231983
- PMCID: PMC11375224
- DOI: 10.1038/s41467-024-51884-8
Cannabidiol ameliorates mitochondrial disease via PPARγ activation in preclinical models
Abstract
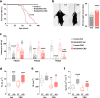
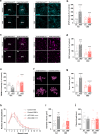
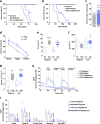
Mutations in mitochondrial energy-producing genes lead to a heterogeneous group of untreatable disorders known as primary mitochondrial diseases (MD). Leigh syndrome (LS) is the most common pediatric MD and is characterized by progressive neuromuscular affectation and premature death. Here, we show that daily cannabidiol (CBD) administration significantly extends lifespan and ameliorates pathology in two LS mouse models, and improves cellular function in fibroblasts from LS patients. CBD delays motor decline and neurodegenerative signs, improves social deficits and breathing abnormalities, decreases thermally induced seizures, and improves neuropathology in affected brain regions. Mechanistically, we identify peroxisome proliferator-activated receptor gamma (PPARγ) as a key nuclear receptor mediating CBD's beneficial effects, while also providing proof of dysregulated PPARγ expression and activity as a common feature in both mouse neurons and fibroblasts from LS patients. Taken together, our results provide the first evidence for CBD as a potential treatment for LS.
© 2024. The Author(s).
Conflict of interest statement
E.P., E.S., and A.Q. are listed as inventors on a patent application submitted by Autonomous University of Barcelona for the use of CBD described in this paper. L.R-P., P.P., C.V., and M.M. are current employees at Minoryx Therapeutics. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- ERC-2014-StG-638106/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- LCF/PR/HR20/52400018./"la Caixa" Foundation (Caixa Foundation)
- H2020-MSCA-IF-2020-101030783,/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- H2020-MSCA-COFUND-2014-665919/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- H2020-MSCA-IF-2014-658352/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

