Tumor-infiltrating nerves functionally alter brain circuits and modulate behavior in a mouse model of head-and-neck cancer
- PMID: 39302290
- PMCID: PMC11415076
- DOI: 10.7554/eLife.97916
Tumor-infiltrating nerves functionally alter brain circuits and modulate behavior in a mouse model of head-and-neck cancer
Abstract
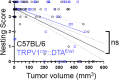
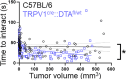
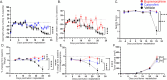
Cancer patients often experience changes in mental health, prompting an exploration into whether nerves infiltrating tumors contribute to these alterations by impacting brain functions. Using a mouse model for head and neck cancer and neuronal tracing, we show that tumor-infiltrating nerves connect to distinct brain areas. The activation of this neuronal circuitry altered behaviors (decreased nest-building, increased latency to eat a cookie, and reduced wheel running). Tumor-infiltrating nociceptor neurons exhibited heightened calcium activity and brain regions receiving these neural projections showed elevated Fos as well as increased calcium responses compared to non-tumor-bearing counterparts. The genetic elimination of nociceptor neurons decreased brain Fos expression and mitigated the behavioral alterations induced by the presence of the tumor. While analgesic treatment restored nesting and cookie test behaviors, it did not fully restore voluntary wheel running indicating that pain is not the exclusive driver of such behavioral shifts. Unraveling the interaction between the tumor, infiltrating nerves, and the brain is pivotal to developing targeted interventions to alleviate the mental health burdens associated with cancer.
Keywords: behavior; brain; cancer biology; head and neck cancer; mouse; neuroscience; sensory nerves; tumor innervation.
Plain language summary
A lot of cancer survivors experience a decline in mental health, persisting often decades after successful treatment. Many factors contribute to this reduced mental well-being, including the physical, emotional and financial stresses they experience. Scientists think that the increased prevalence of mental health disorders among cancer patients and survivors may also be linked to the cancer itself. Previous research has shown that most tumors, in particular in melanomas, cervical and ovarian cancers, and head and neck cancers, contain sensory nerves that sense thermal, mechanical and chemical changes and so alert an organism about a potential danger, such as extreme temperature, pressure, changes in pH or inflammation. To investigate whether these nerves contribute to the worsened mental health of cancer patients, Barr, Walz et al. studied male mice with tumors growing in their mouths, mimicking the disease of patients with head and neck cancers. The mice with tumors exhibited several altered behaviors linked to their well-being, suggesting that they had reduced overall health compared to mice without tumors. For example, they were less inclined to build nests, accept treats or run on a wheel. Next, Barr, Walz et al. injected a fluorescent dye into the tumors to label the nerves inside the cancerous growths. Fluorescence microscopy and imaging studies revealed that, days later, the dye had traveled to multiple regions of the brain, indicating that the nerves in the tumors had connected to a preexisting nerve circuit that included these brain regions. Further experiments revealed that the nerve cells in these brain regions were more active in mice with tumors and had different functional properties compared to mice without tumors. Removing the connecting nerves either genetically or with a drug improved all the behaviors of the mice with tumors. Treating the mice with painkillers also improved some but not all of their behaviors, indicating that pain is not the exclusive driver of such behavioral shifts. These two experiments suggest that the nerves from the tumors relay information about pain to the brain and contribute to reduced well-being of the mice. Further studies will test whether these tumor-brain connections also contribute to behavioral changes in mice with other types of cancer. The data suggest that disrupting the neural connections between a tumor and the brain may improve the mental health of patients with cancer, but more research is needed to establish this link.
© 2024, Barr, Walz et al.
Conflict of interest statement
JB, AW, AR, MA, SB, EV, WS, RD, ST, PV No competing interests declared
Figures





Update of
-
Tumor-infiltrating nerves functionally alter brain circuits and modulate behavior in a male mouse model of head-and-neck cancer.bioRxiv [Preprint]. 2024 Jun 7:2023.10.18.562990. doi: 10.1101/2023.10.18.562990. bioRxiv. 2024. Update in: Elife. 2024 Sep 20;13:RP97916. doi: 10.7554/eLife.97916. PMID: 37905135 Free PMC article. Updated. Preprint.
References
-
- Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, Anfossi S, Osman AA, Cai Y, Wang R, Knutsen E, Shimizu M, Ivan C, Rao X, Wang J, Silverman DA, Tam S, Zhao M, Caulin C, Zinger A, Tasciotti E, Dougherty PM, El-Naggar A, Calin GA, Myers JN. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature. 2020;578:449–454. doi: 10.1038/s41586-020-1996-3. - DOI - PMC - PubMed
-
- Balood M, Ahmadi M, Eichwald T, Ahmadi A, Majdoubi A, Roversi K, Roversi K, Lucido CT, Restaino AC, Huang S, Ji L, Huang K-C, Semerena E, Thomas SC, Trevino AE, Merrison H, Parrin A, Doyle B, Vermeer DW, Spanos WC, Williamson CS, Seehus CR, Foster SL, Dai H, Shu CJ, Rangachari M, Thibodeau J, V Del Rincon S, Drapkin R, Rafei M, Ghasemlou N, Vermeer PD, Woolf CJ, Talbot S. Nociceptor neurons affect cancer immunosurveillance. Nature. 2022;611:405–412. doi: 10.1038/s41586-022-05374-w. - DOI - PMC - PubMed
-
- Barr JL, Kruse A, Restaino AC, Tulina N, Stuckelberger S, Vermeer SJ, Williamson CS, Vermeer DW, Madeo M, Stamp J, Bell M, Morgan M, Yoon J-Y, Mitchell MA, Budina A, Omran DK, Schwartz LE, Drapkin R, Vermeer PD. Intra-Tumoral Nerve-Tracing in a novel syngeneic model of high-grade serous ovarian carcinoma. Cells. 2021;10:3491. doi: 10.3390/cells10123491. - DOI - PMC - PubMed
MeSH terms
Associated data
- Actions
Grants and funding
- 5R01DE032712-02/DE/NIDCR NIH HHS/United States
- R01 CA193522/NH/NIH HHS/United States
- 461275/CAPMC/ CIHR/Canada
- 5P20GM103548/GM/NIGMS NIH HHS/United States
- R21 NS130712/NS/NINDS NIH HHS/United States
- 162211/CAPMC/ CIHR/Canada
- 1R37CA242006-01A1/CA/NCI NIH HHS/United States
- P30 GM145398/GM/NIGMS NIH HHS/United States
- R01 CA193522/CA/NCI NIH HHS/United States
- Discovery Award/Stiefel family
- P20 GM103548/GM/NIGMS NIH HHS/United States
- 461274/CAPMC/ CIHR/Canada
- U54 GM128729/GM/NIGMS NIH HHS/United States
- R21 NS130712/NH/NIH HHS/United States
- R01 DE032712/DE/NIDCR NIH HHS/United States
- R37 CA242006/CA/NCI NIH HHS/United States
- MDACC/Moonshot Research and Development Program
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

