Epididymis-specific RNase A family genes regulate fertility and small RNA processing
- PMID: 39476961
- PMCID: PMC11647505
- DOI: 10.1016/j.jbc.2024.107933
Epididymis-specific RNase A family genes regulate fertility and small RNA processing
Abstract
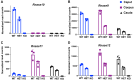
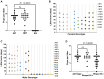
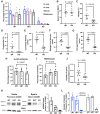
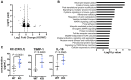
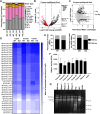
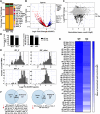
Sperm small RNAs are implicated in intergenerational transmission of paternal environmental effects. Small RNAs generated by the cleavage of tRNAs, known as tRNA fragments (tRFs) or tRNA-derived RNAs (tDRs or tsRNAs), are an abundant class of RNAs in mature sperm and can be modulated by environmental conditions. The biogenesis of tRFs in the male reproductive tract remains poorly understood. Angiogenin, a member of the ribonuclease A superfamily (RNase A), cleaves tRNAs to generate tRFs in response to cellular stress. Four paralogs of Angiogenin, namely Rnase9, Rnase10, Rnase11, and Rnase12, are specifically expressed in the epididymis-a long, convoluted tubule where sperm mature and acquire fertility and motility. Here, by generating mice deleted for all four genes (Rnase9-12-/-, termed "KO" for Knock Out), we report that these genes regulate fertility and small RNA levels. KO male mice are sterile; KO sperm fertilized oocytes in vitro but failed to efficiently fertilize oocytes in vivo due to an inability of sperm to pass through the utero-tubular junction. Intriguingly, there were decreased levels of tRFs and rRNAs (rRNA-derived small RNAs or rsRNAs) in the KO epididymis and epididymal luminal fluid, although RNases 9-12 did not show ribonucleolytic activity in vitro. Importantly, KO sperm showed a dramatic decrease in the levels of tRFs, demonstrating a role of epididymis-specific Rnase9-12 genes in regulating sperm small RNA composition. Together, our results reveal an unexpected role of four epididymis-specific noncanonical ribonuclease A family genes in regulating fertility and small RNA processing.
Keywords: epididymis; fertility; reproduction; ribonucleases; small RNAs; sperm.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interests The authors declare that they have no conflicts of interests with the contents of this article.
Figures

Update of
-
Epididymis-specific RNase A family genes regulate fertility and small RNA processing.bioRxiv [Preprint]. 2024 Aug 27:2024.08.26.608813. doi: 10.1101/2024.08.26.608813. bioRxiv. 2024. Update in: J Biol Chem. 2024 Dec;300(12):107933. doi: 10.1016/j.jbc.2024.107933. PMID: 39253511 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

