Targeting c-Myc transactivation by LMNA inhibits tRNA processing essential for malate-aspartate shuttle and tumour progression
- PMID: 38769668
- PMCID: PMC11106511
- DOI: 10.1002/ctm2.1680
Targeting c-Myc transactivation by LMNA inhibits tRNA processing essential for malate-aspartate shuttle and tumour progression
Abstract
Background: A series of studies have demonstrated the emerging involvement of transfer RNA (tRNA) processing during the progression of tumours. Nevertheless, the roles and regulating mechanisms of tRNA processing genes in neuroblastoma (NB), the prevalent malignant tumour outside the brain in children, are yet unknown.
Methods: Analysis of multi-omics results was conducted to identify crucial regulators of downstream tRNA processing genes. Co-immunoprecipitation and mass spectrometry methods were utilised to measure interaction between proteins. The impact of transcriptional regulators on expression of downstream genes was measured by dual-luciferase reporter, chromatin immunoprecipitation, western blotting and real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) methods. Studies have been conducted to reveal impact and mechanisms of transcriptional regulators on biological processes of NB. Survival differences were analysed using the log-rank test.
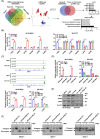
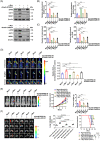
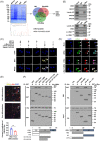
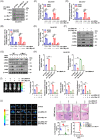
Results: c-Myc was identified as a transcription factor driving tRNA processing gene expression and subsequent malate-aspartate shuttle (MAS) in NB cells. Mechanistically, c-Myc directly promoted the expression of glutamyl-prolyl-tRNA synthetase (EPRS) and leucyl-tRNA synthetase (LARS), resulting in translational up-regulation of glutamic-oxaloacetic transaminase 1 (GOT1) as well as malate dehydrogenase 1 (MDH1) via inhibiting general control nonrepressed 2 or activating mechanistic target of rapamycin signalling. Meanwhile, lamin A (LMNA) inhibited c-Myc transactivation via physical interaction, leading to suppression of MAS, aerobic glycolysis, tumourigenesis and aggressiveness. Pre-clinically, lobeline was discovered as a LMNA-binding compound to facilitate its interaction with c-Myc, which inhibited aminoacyl-tRNA synthetase expression, MAS and tumour progression of NB, as well as growth of organoid derived from c-Myc knock-in mice. Low levels of LMNA or elevated expression of c-Myc, EPRS, LARS, GOT1 or MDH1 were linked to a worse outcome and a shorter survival time of clinical NB patients.
Conclusions: These results suggest that targeting c-Myc transactivation by LMNA inhibits tRNA processing essential for MAS and tumour progression.
Keywords: c‐Myc; lamin A; malate‐aspartate shuttle; neuroblastoma; transfer RNA processing; tumour progression.
© 2024 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures



References
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
