BET bromodomain inhibition as a therapeutic strategy to target c-Myc
- PMID: 21889194
- PMCID: PMC3187920
- DOI: 10.1016/j.cell.2011.08.017
BET bromodomain inhibition as a therapeutic strategy to target c-Myc
Abstract
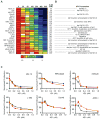
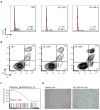
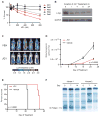
MYC contributes to the pathogenesis of a majority of human cancers, yet strategies to modulate the function of the c-Myc oncoprotein do not exist. Toward this objective, we have targeted MYC transcription by interfering with chromatin-dependent signal transduction to RNA polymerase, specifically by inhibiting the acetyl-lysine recognition domains (bromodomains) of putative coactivator proteins implicated in transcriptional initiation and elongation. Using a selective small-molecule bromodomain inhibitor, JQ1, we identify BET bromodomain proteins as regulatory factors for c-Myc. BET inhibition by JQ1 downregulates MYC transcription, followed by genome-wide downregulation of Myc-dependent target genes. In experimental models of multiple myeloma, a Myc-dependent hematologic malignancy, JQ1 produces a potent antiproliferative effect associated with cell-cycle arrest and cellular senescence. Efficacy of JQ1 in three murine models of multiple myeloma establishes the therapeutic rationale for BET bromodomain inhibition in this disease and other malignancies characterized by pathologic activation of c-Myc.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
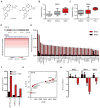
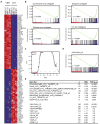
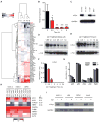
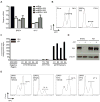
Comment in
-
Targeting MYC? You BET.Nat Rev Cancer. 2011 Sep 23;11(10):693. doi: 10.1038/nrc3147. Nat Rev Cancer. 2011. PMID: 21941280 No abstract available.
-
Targeting MYC? You BET.Nat Rev Drug Discov. 2011 Sep 30;10(10):732-3. doi: 10.1038/nrd3569. Nat Rev Drug Discov. 2011. PMID: 21959283 No abstract available.
References
-
- Amati B, Brooks MW, Levy N, Littlewood TD, Evan GI, Land H. Oncogenic activity of the c-Myc protein requires dimerization with Max. Cell. 1993;72:233–245. - PubMed
-
- Bidwell GL, 3rd, Davis AN, Raucher D. Targeting a c-Myc inhibitory polypeptide to specific intracellular compartments using cell penetrating peptides. J Control Release. 2009;135:2–10. - PubMed
-
- Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251:1211–1217. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

