E-cadherin is required for intestinal morphogenesis in the mouse
- PMID: 22766025
- PMCID: PMC3455111
- DOI: 10.1016/j.ydbio.2012.06.005
E-cadherin is required for intestinal morphogenesis in the mouse
Abstract
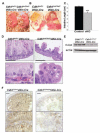
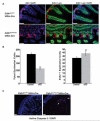
E-cadherin, the primary epithelial adherens junction protein, has been implicated as playing a critical role in nucleating formation of adherens junctions, tight junctions, and desmosomes. In addition to its role in maintaining structural tissue integrity, E-cadherin has also been suggested as an important modulator of cell signaling via interactions with its cytoplasmic binding partners, catenins, as well as with growth factor receptors. Therefore, we proposed that loss of E-cadherin from the developing mouse intestinal epithelium would disrupt intestinal epithelial morphogenesis and function. To test this hypothesis, we used a conditional knockout approach to eliminate E-cadherin specifically in the intestinal epithelium during embryonic development. We found that E-cadherin conditional knockout mice failed to survive, dying within the first 24 hours of birth. Examination of intestinal architecture at E18.5 demonstrated severe disruption to intestinal morphogenesis in animals lacking E-cadherin in the epithelium of the small intestine. We observed changes in epithelial cell shape as well as in the morphology of villi. Although junctional complexes were evident, junctions were abnormal, and barrier function was compromised in E-cadherin mutant intestine. We also identified changes in the epithelial cell populations present in E-cadherin conditional knockout animals. The number of proliferating cells was increased, whereas the number of enterocytes was decreased. Although Wnt/β-catenin target mRNAs were more abundant in mutants compared with controls, the amount of nuclear activated β-catenin protein was dramatically lower in mutants compared with controls. In summary, our data demonstrate that E-cadherin is essential for intestinal epithelial morphogenesis and homeostasis during embryonic development.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures






References
-
- Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 2007.
-
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115:53–62. - PubMed
-
- Cali G, Zannini M, Rubini P, Tacchetti C, D’Andrea B, Affuso A, Wintermantel T, Boussadia O, Terracciano D, Silberschmidt D, Amendola E, De Felice M, Schutz G, Kemler R, Di Lauro R, Nitsch L. Conditional inactivation of the E-cadherin gene in thyroid follicular cells affects gland development but does not impair junction formation. Endocrinology. 2007;148:2737–2746. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

