Generation of oligodendroglial cells by direct lineage conversion
- PMID: 23584610
- PMCID: PMC3677690
- DOI: 10.1038/nbt.2564
Generation of oligodendroglial cells by direct lineage conversion
Abstract
Transplantation of oligodendrocyte precursor cells (OPCs) is a promising potential therapeutic strategy for diseases affecting myelin. However, the derivation of engraftable OPCs from human pluripotent stem cells has proven difficult and primary OPCs are not readily available. Here we report the generation of induced OPCs (iOPCs) by direct lineage conversion. Forced expression of the three transcription factors Sox10, Olig2 and Zfp536 was sufficient to reprogram mouse and rat fibroblasts into iOPCs with morphologies and gene expression signatures resembling primary OPCs. More importantly, iOPCs gave rise to mature oligodendrocytes that could ensheath multiple host axons when co-cultured with primary dorsal root ganglion cells and formed myelin after transplantation into shiverer mice. We propose direct lineage reprogramming as a viable alternative approach for the generation of OPCs for use in disease modeling and regenerative medicine.
Figures
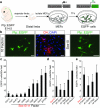
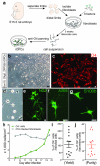

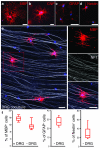
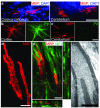
Comment in
-
iOPs: a new tool for studying myelin pathologies?Cell Stem Cell. 2013 May 2;12(5):503-4. doi: 10.1016/j.stem.2013.04.021. Cell Stem Cell. 2013. PMID: 23642358
-
Neural repair and rehabilitation: Fibroblast-derived OPCs--hope for remyelination therapy?Nat Rev Neurol. 2013 Jun;9(6):299. doi: 10.1038/nrneurol.2013.85. Epub 2013 May 7. Nat Rev Neurol. 2013. PMID: 23649101 No abstract available.
-
White matter from fibroblasts.Nat Biotechnol. 2013 May;31(5):412-3. doi: 10.1038/nbt.2570. Nat Biotechnol. 2013. PMID: 23657393 Free PMC article.
-
Changing one cell type into another.Nat Methods. 2013 Jun;10(6):459. doi: 10.1038/nmeth.2500. Nat Methods. 2013. PMID: 23866329 No abstract available.
References
-
- Potter GB, Rowitch DH, Petryniak MA. Myelin restoration: progress and prospects for human cell replacement therapies. Arch Immunol Ther Exp (Warsz) 2011;59:179–193. - PubMed
-
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. - PubMed
-
- Franklin RJ, Ffrench-Constant C. Stem cell treatments and multiple sclerosis. BMJ. 2010;340:c1387. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

