Identification of novel molecular markers through transcriptomic analysis in human fetal and adult corneal endothelial cells
- PMID: 23257286
- PMCID: PMC3596846
- DOI: 10.1093/hmg/dds527
Identification of novel molecular markers through transcriptomic analysis in human fetal and adult corneal endothelial cells
Abstract
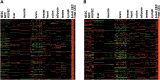
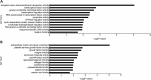
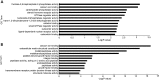
The corneal endothelium is composed of a monolayer of corneal endothelial cells (CECs), which is essential for maintaining corneal transparency. To better characterize CECs in different developmental stages, we profiled mRNA transcriptomes in human fetal and adult corneal endothelium with the goal to identify novel molecular markers in these cells. By comparing CECs with 12 other tissue types, we identified 245 and 284 signature genes that are highly expressed in fetal and adult CECs, respectively. Functionally, these genes are enriched in pathways characteristic of CECs, including inorganic anion transmembrane transporter, extracellular matrix structural constituent and cyclin-dependent protein kinase inhibitor activity. Importantly, several of these genes are disease target genes in hereditary corneal dystrophies, consistent with their functional significance in CEC physiology. We also identified stage-specific markers associated with CEC development, such as specific members in the transforming growth factor beta and Wnt signaling pathways only expressed in fetal, but not in adult CECs. Lastly, by the immunohistochemistry of ocular tissues, we demonstrated the unique protein localization for Wnt5a, S100A4, S100A6 and IER3, the four novel markers for fetal and adult CECs. The identification of a new panel of stage-specific markers for CECs would be very useful for characterizing CECs derived from stem cells or ex vivo expansion for cell replacement therapy.
Figures


References
-
- Koizumi N., Okumura N., Kinoshita S. Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models. Exp. Eye Res. 2012;95:60–67. - PubMed
-
- Peh G.S., Beuerman R.W., Colman A., Tan D.T., Mehta J.S. Human corneal endothelial cell expansion for corneal endothelium transplantation: an overview. Transplantation. 2011;91:811–819. - PubMed
-
- Weiss J.S., Moller H.U., Lisch W., Kinoshita S., Aldave A.J., Belin M.W., Kivela T., Busin M., Munier F.L., Seitz B., et al. [The IC3D classification of the corneal dystrophies] Klin. Monbl. Augenheilkd. 2011;228(Suppl. 1):S1–S39. - PubMed
-
- Ghosheh F.R., Cremona F.A., Rapuano C.J., Cohen E.J., Ayres B.D., Hammersmith K.M., Raber I.M., Laibson P.R. Trends in penetrating keratoplasty in the United States 1980–2005. Int. Ophthalmol. 2008;28:147–153. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

