Bcl11a controls Flt3 expression in early hematopoietic progenitors and is required for pDC development in vivo
- PMID: 23741395
- PMCID: PMC3669380
- DOI: 10.1371/journal.pone.0064800
Bcl11a controls Flt3 expression in early hematopoietic progenitors and is required for pDC development in vivo
Abstract
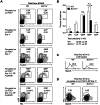
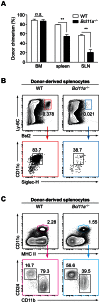
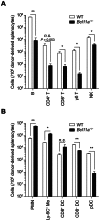
Bcl11a is a transcription factor known to regulate lymphoid and erythroid development. Recent bioinformatic analysis of global gene expression patterns has suggested a role for Bcl11a in the development of dendritic cell (DC) lineages. We tested this hypothesis by analyzing the development of DC and other lineages in Bcl11a (-/-) mice. We found that Bcl11a was required for expression of IL-7 receptor (IL-7R) and Flt3 in early hematopoietic progenitor cells. In addition, we found severely decreased numbers of plasmacytoid dendritic cells (pDCs) in Bcl11a (-/-) fetal livers and in the bone marrow of Bcl11a (-/-) fetal liver chimeras. Moreover, Bcl11a (-/-) cells showed severely impaired in vitro development of Flt3L-derived pDCs and classical DCs (cDCs). In contrast, we found normal in vitro development of DCs from Bcl11a (-/-) fetal liver cells treated with GM-CSF. These results suggest that the persistent cDC development observed in Bcl11a (-/-) fetal liver chimeras reflects derivation from a Bcl11a- and Flt3-independent pathway in vivo.
Conflict of interest statement
Figures




References
-
- Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, et al. (2006) Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol 7: 663–671. - PubMed
-
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, et al. (2007) Identification of clonogenic common Flt3+ M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nature 8: 1207–1216. - PubMed
-
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, et al. (2007) Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol 8: 1217–1226. - PubMed
-
- Wu L, Nichogiannopoulou A, Shortman K, Georgopoulos K (1997) Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity 7: 483–492. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

