Whole-genome analysis of 5-hydroxymethylcytosine and 5-methylcytosine at base resolution in the human brain
- PMID: 24594098
- PMCID: PMC4053808
- DOI: 10.1186/gb-2014-15-3-r49
Whole-genome analysis of 5-hydroxymethylcytosine and 5-methylcytosine at base resolution in the human brain
Abstract
Background: 5-methylcytosine (mC) can be oxidized by the tet methylcytosine dioxygenase (Tet) family of enzymes to 5-hydroxymethylcytosine (hmC), which is an intermediate of mC demethylation and may also be a stable epigenetic modification that influences chromatin structure. hmC is particularly abundant in mammalian brains but its function is currently unknown. A high-resolution hydroxymethylome map is required to fully understand the function of hmC in the human brain.
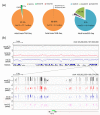
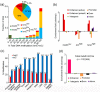
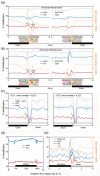
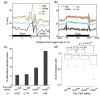
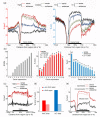
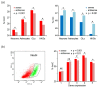
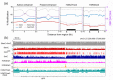
Results: We present genome-wide and single-base resolution maps of hmC and mC in the human brain by combined application of Tet-assisted bisulfite sequencing and bisulfite sequencing. We demonstrate that hmCs increase markedly from the fetal to the adult stage, and in the adult brain, 13% of all CpGs are highly hydroxymethylated with strong enrichment at genic regions and distal regulatory elements. Notably, hmC peaks are identified at the 5'splicing sites at the exon-intron boundary, suggesting a mechanistic link between hmC and splicing. We report a surprising transcription-correlated hmC bias toward the sense strand and an mC bias toward the antisense strand of gene bodies. Furthermore, hmC is negatively correlated with H3K27me3-marked and H3K9me3-marked repressive genomic regions, and is more enriched at poised enhancers than active enhancers.
Conclusions: We provide single-base resolution hmC and mC maps in the human brain and our data imply novel roles of hmC in regulating splicing and gene expression. Hydroxymethylation is the main modification status for a large portion of CpGs situated at poised enhancers and actively transcribed regions, suggesting its roles in epigenetic tuning at these regions.
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

