Protein microarray analysis reveals BAFF-binding autoantibodies in systemic lupus erythematosus
- PMID: 24270423
- PMCID: PMC3859403
- DOI: 10.1172/JCI70231
Protein microarray analysis reveals BAFF-binding autoantibodies in systemic lupus erythematosus
Abstract
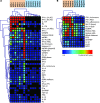
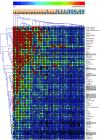
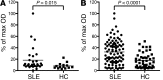
Autoantibodies against cytokines, chemokines, and growth factors inhibit normal immunity and are implicated in inflammatory autoimmune disease and diseases of immune deficiency. In an effort to evaluate serum from autoimmune and immunodeficient patients for Abs against cytokines, chemokines, and growth factors in a high-throughput and unbiased manner, we constructed a multiplex protein microarray for detection of serum factor-binding Abs and used the microarray to detect autoantibody targets in SLE. We designed a nitrocellulose-surface microarray containing human cytokines, chemokines, and other circulating proteins and demonstrated that the array permitted specific detection of serum factor-binding probes. We used the arrays to detect previously described autoantibodies against cytokines in samples from individuals with autoimmune polyendocrine syndrome type 1 and chronic mycobacterial infection. Serum profiling from individuals with SLE revealed that among several targets, elevated IgG autoantibody reactivity to B cell-activating factor (BAFF) was associated with SLE compared with control samples. BAFF reactivity correlated with the severity of disease-associated features, including IFN-α-driven SLE pathology. Our results showed that serum factor protein microarrays facilitate detection of autoantibody reactivity to serum factors in human samples and that BAFF-reactive autoantibodies may be associated with an elevated inflammatory disease state within the spectrum of SLE.
Figures


Comment in
-
BAFF-ling autoantibodies.J Clin Invest. 2013 Dec;123(12):5006-8. doi: 10.1172/JCI73166. Epub 2013 Nov 25. J Clin Invest. 2013. PMID: 24270413 Free PMC article.
References
-
- Patel SY, et al. Anti-IFN-γ autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. 2005;175(7):4769–4776. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

