REST and stress resistance in ageing and Alzheimer's disease
- PMID: 24670762
- PMCID: PMC4110979
- DOI: 10.1038/nature13163
REST and stress resistance in ageing and Alzheimer's disease
Erratum in
-
Addendum: REST and stress resistance in ageing and Alzheimer's disease.Nature. 2016 Dec 15;540(7633):470. doi: 10.1038/nature20579. Epub 2016 Nov 16. Nature. 2016. PMID: 27851730 No abstract available.
Abstract
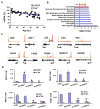
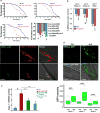
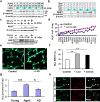
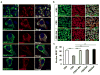
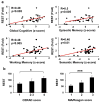
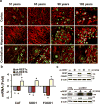
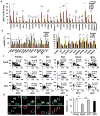
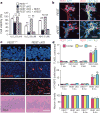
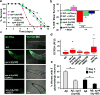
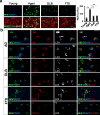
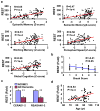
Human neurons are functional over an entire lifetime, yet the mechanisms that preserve function and protect against neurodegeneration during ageing are unknown. Here we show that induction of the repressor element 1-silencing transcription factor (REST; also known as neuron-restrictive silencer factor, NRSF) is a universal feature of normal ageing in human cortical and hippocampal neurons. REST is lost, however, in mild cognitive impairment and Alzheimer's disease. Chromatin immunoprecipitation with deep sequencing and expression analysis show that REST represses genes that promote cell death and Alzheimer's disease pathology, and induces the expression of stress response genes. Moreover, REST potently protects neurons from oxidative stress and amyloid β-protein toxicity, and conditional deletion of REST in the mouse brain leads to age-related neurodegeneration. A functional orthologue of REST, Caenorhabditis elegans SPR-4, also protects against oxidative stress and amyloid β-protein toxicity. During normal ageing, REST is induced in part by cell non-autonomous Wnt signalling. However, in Alzheimer's disease, frontotemporal dementia and dementia with Lewy bodies, REST is lost from the nucleus and appears in autophagosomes together with pathological misfolded proteins. Finally, REST levels during ageing are closely correlated with cognitive preservation and longevity. Thus, the activation state of REST may distinguish neuroprotection from neurodegeneration in the ageing brain.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Comment in
-
Alzheimer's disease: A protective factor for the ageing brain.Nature. 2014 Mar 27;507(7493):439-40. doi: 10.1038/nature13214. Epub 2014 Mar 19. Nature. 2014. PMID: 24670758 No abstract available.
-
Neurodegeneration: Ageing neurons need REST.Nat Rev Neurosci. 2014 May;15(5):279. doi: 10.1038/nrn3728. Epub 2014 Apr 3. Nat Rev Neurosci. 2014. PMID: 24675782 No abstract available.
-
REST as a new therapeutic target for neurodegenerative disorders.Mov Disord. 2014 Jun;29(7):869. doi: 10.1002/mds.25910. Epub 2014 May 15. Mov Disord. 2014. PMID: 24833024 No abstract available.
-
Alternative REST Splicing Underappreciated.eNeuro. 2018 Oct 1;5(5):ENEURO.0034-18.2018. doi: 10.1523/ENEURO.0034-18.2018. eCollection 2018 Sep-Oct. eNeuro. 2018. PMID: 30280122 Free PMC article. No abstract available.
References
-
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8:671–684. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- DP1OD006849/OD/NIH HHS/United States
- R01AG26651/AG/NIA NIH HHS/United States
- T32 AG000222/AG/NIA NIH HHS/United States
- R01 GM105853/GM/NIGMS NIH HHS/United States
- R01 GM072551/GM/NIGMS NIH HHS/United States
- P01 AG027916/AG/NIA NIH HHS/United States
- R01AG15819/AG/NIA NIH HHS/United States
- DP1 AG044161/AG/NIA NIH HHS/United States
- P01AG27916/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- R01AG17917/AG/NIA NIH HHS/United States
- R01GM072551/GM/NIGMS NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- DP1 OD006849/OD/NIH HHS/United States
- P30AG10161/AG/NIA NIH HHS/United States
- R01 AG026651/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

