The cellular and molecular origin of tumor-associated macrophages
- PMID: 24812208
- PMCID: PMC4204732
- DOI: 10.1126/science.1252510
The cellular and molecular origin of tumor-associated macrophages
Abstract
Long recognized as an evolutionarily ancient cell type involved in tissue homeostasis and immune defense against pathogens, macrophages are being rediscovered as regulators of several diseases, including cancer. Here we show that in mice, mammary tumor growth induces the accumulation of tumor-associated macrophages (TAMs) that are phenotypically and functionally distinct from mammary tissue macrophages (MTMs). TAMs express the adhesion molecule Vcam1 and proliferate upon their differentiation from inflammatory monocytes, but do not exhibit an "alternatively activated" phenotype. TAM terminal differentiation depends on the transcriptional regulator of Notch signaling, RBPJ; and TAM, but not MTM, depletion restores tumor-infiltrating cytotoxic T cell responses and suppresses tumor growth. These findings reveal the ontogeny of TAMs and a discrete tumor-elicited inflammatory response, which may provide new opportunities for cancer immunotherapy.
Copyright © 2014, American Association for the Advancement of Science.
Figures
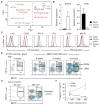


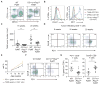
Comment in
-
Cancer immunology. Identifying the infiltrators.Science. 2014 May 23;344(6186):801-2. doi: 10.1126/science.1255117. Science. 2014. PMID: 24855239 No abstract available.
-
The origin and function of tumor-associated macrophages.Cell Mol Immunol. 2015 Jan;12(1):1-4. doi: 10.1038/cmi.2014.83. Epub 2014 Sep 15. Cell Mol Immunol. 2015. PMID: 25220733 Free PMC article. No abstract available.
References
-
- Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012 Apr 6;336:86. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

