Two nested developmental waves demarcate a compartment boundary in the mouse lung
- PMID: 24879355
- PMCID: PMC4115076
- DOI: 10.1038/ncomms4923
Two nested developmental waves demarcate a compartment boundary in the mouse lung
Abstract
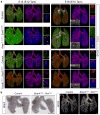
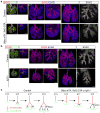
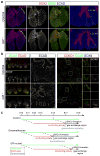
The lung is a branched tubular network with two distinct compartments--the proximal conducting airways and the peripheral gas exchange region--separated by a discrete boundary termed the bronchoalveolar duct junction (BADJ). Here we image the developing mouse lung in three-dimensions (3D) and show that two nested developmental waves demarcate the BADJ under the control of a global hormonal signal. A first wave of branching morphogenesis progresses throughout embryonic development, generating branches for both compartments. A second wave of conducting airway differentiation follows the first wave but terminates earlier, specifying the proximal compartment and setting the BADJ. The second wave is terminated by a glucocorticoid signalling: premature activation or loss of glucocorticoid signalling causes a proximal or distal shift, respectively, in BADJ location. The results demonstrate a new mechanism of boundary formation in complex, 3D organs and provide new insights into glucocorticoid therapies for lung defects in premature birth.
Conflict of interest statement
Competing financial interests
The authors declare no conflict of interest.
Figures




References
-
- Weibel ER. What makes a good lung? Swiss Med Wkly. 2009;139:375–86. - PubMed
-
- Bal HS, Ghoshal NG. Morphology of the terminal bronchiolar region of common laboratory mammals. Lab Anim. 1988;22:76–82. - PubMed
-
- Haefeli-Bleuer B, Weibel ER. Morphometry of the human pulmonary acinus. Anat Rec. 1988;220:401–14. - PubMed
-
- Ten Have-Opbroek AA. The development of the lung in mammals: an analysis of concepts and findings. Am J Anat. 1981;162:201–19. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

