Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness
- PMID: 25274297
- PMCID: PMC4214913
- DOI: 10.1038/nature13823
Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness
Abstract
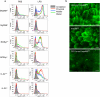
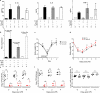
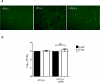
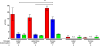
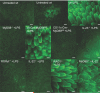
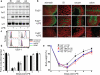
Systemic infection induces conserved physiological responses that include both resistance and 'tolerance of infection' mechanisms. Temporary anorexia associated with an infection is often beneficial, reallocating energy from food foraging towards resistance to infection or depriving pathogens of nutrients. However, it imposes a stress on intestinal commensals, as they also experience reduced substrate availability; this affects host fitness owing to the loss of caloric intake and colonization resistance (protection from additional infections). We hypothesized that the host might utilize internal resources to support the gut microbiota during the acute phase of the disease. Here we show that systemic exposure to Toll-like receptor (TLR) ligands causes rapid α(1,2)-fucosylation of small intestine epithelial cells (IECs) in mice, which requires the sensing of TLR agonists, as well as the production of interleukin (IL)-23 by dendritic cells, activation of innate lymphoid cells and expression of fucosyltransferase 2 (Fut2) by IL-22-stimulated IECs. Fucosylated proteins are shed into the lumen and fucose is liberated and metabolized by the gut microbiota, as shown by reporter bacteria and community-wide analysis of microbial gene expression. Fucose affects the expression of microbial metabolic pathways and reduces the expression of bacterial virulence genes. It also improves host tolerance of the mild pathogen Citrobacter rodentium. Thus, rapid IEC fucosylation appears to be a protective mechanism that utilizes the host's resources to maintain host-microbial interactions during pathogen-induced stress.
Figures



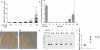



Comment in
-
Immunology: Starve a fever, feed the microbiota.Nature. 2014 Oct 30;514(7524):576-7. doi: 10.1038/nature13756. Epub 2014 Oct 1. Nature. 2014. PMID: 25274298 No abstract available.
-
Microbiota: support your friends to resist your enemies.Nat Rev Immunol. 2014 Nov;14(11):715. doi: 10.1038/nri3765. Nat Rev Immunol. 2014. PMID: 25341430 No abstract available.
References
-
- Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30:271–294. doi:10.1146/annurev-immunol-020711-075030. - PubMed
-
- Murray MJ, Murray AB. Anorexia of infection as a mechanism of host defense. Am J Clin Nutr. 1979;32:593–596. - PubMed
-
- Kyriazakis II, Tolkamp BJ, Hutchings MR. Towards a functional explanation for the occurrence of anorexia during parasitic infections. Animal behaviour. 1998;56:265–274. doi:10.1006/anbe.1998.0761. - PubMed
-
- Exton MS. Infection-induced anorexia: active host defence strategy. Appetite. 1997;29:369–383. doi:10.1006/appe.1997.0116. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- UL1 TR000430/TR/NCATS NIH HHS/United States
- P30 DK042086/DK/NIDDK NIH HHS/United States
- P50 GM068763/GM/NIGMS NIH HHS/United States
- R01 AI090084/AI/NIAID NIH HHS/United States
- AI96706/AI/NIAID NIH HHS/United States
- R41 AI096706/AI/NIAID NIH HHS/United States
- T32 AI007090/AI/NIAID NIH HHS/United States
- AI42135/AI/NIAID NIH HHS/United States
- T32 AI065382/AI/NIAID NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- DK42086/DK/NIDDK NIH HHS/United States
- R01 AI095706/AI/NIAID NIH HHS/United States
- R01 AI042135/AI/NIAID NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

