Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation
- PMID: 25733684
- PMCID: PMC4405637
- DOI: 10.1128/MCB.01321-14
Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation
Abstract
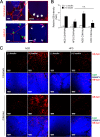
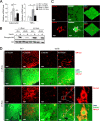
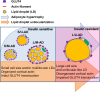
In obesity, adipocyte hypertrophy and proinflammatory responses are closely associated with the development of insulin resistance in adipose tissue. However, it is largely unknown whether adipocyte hypertrophy per se might be sufficient to provoke insulin resistance in obese adipose tissue. Here, we demonstrate that lipid-overloaded hypertrophic adipocytes are insulin resistant independent of adipocyte inflammation. Treatment with saturated or monounsaturated fatty acids resulted in adipocyte hypertrophy, but proinflammatory responses were observed only in adipocytes treated with saturated fatty acids. Regardless of adipocyte inflammation, hypertrophic adipocytes with large and unilocular lipid droplets exhibited impaired insulin-dependent glucose uptake, associated with defects in GLUT4 trafficking to the plasma membrane. Moreover, Toll-like receptor 4 mutant mice (C3H/HeJ) with high-fat-diet-induced obesity were not protected against insulin resistance, although they were resistant to adipose tissue inflammation. Together, our in vitro and in vivo data suggest that adipocyte hypertrophy alone may be crucial in causing insulin resistance in obesity.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






References
-
- Martinsson A. 1969. Hypertrophy and hyperplasia of human adipose tissue in obesity. Pol Arch Med Wewn 42:481–486. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
