Steroid Signaling Establishes a Female Metabolic State and Regulates SREBP to Control Oocyte Lipid Accumulation
- PMID: 25802149
- PMCID: PMC6894397
- DOI: 10.1016/j.cub.2015.02.019
Steroid Signaling Establishes a Female Metabolic State and Regulates SREBP to Control Oocyte Lipid Accumulation
Abstract
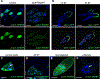
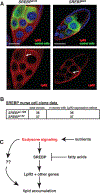
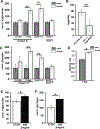
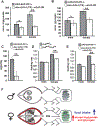
Disruptions in energy homeostasis severely affect reproduction in many organisms and are linked to several reproductive disorders in humans. As a result, understanding the mechanisms that control nutrient accumulation in the oocyte will provide valuable insights into the links between metabolic disease and reproductive dysfunction. We show that the steroid hormone ecdysone functions in Drosophila to control lipid metabolism and support oocyte production. First, local EcR-mediated signaling induces a stage-specific accumulation of lipids in stage-10 oocytes. EcR induces lipid accumulation by promoting the activation of the lipogenic transcription factor SREBP and by controlling the expression of the low-density lipoprotein (LDL) receptor homolog, LpR2. Second, global signaling via the ecdysone receptor, EcR, establishes a female metabolic state and promotes whole-body triglyceride and glycogen storage at high levels. EcR acts in the CNS to mediate these effects, in part by promoting higher levels of feeding in females. Thus, ecdysone functions at two levels to support reproduction: first by inducing lipid accumulation in the late stages of oocyte development and second by providing a signal that coordinates lipid metabolism in the germline with whole-animal lipid homeostasis. Ecdysone regulation allows females to assess the demands of oogenesis and alter their behavior and metabolic state to support the biosynthetic requirements of oocyte production.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures



References
-
- Orbetzova MM, Kamenov ZA, Kolarov GB, Orbetzova VT, Genchev GD, Genov NS, and Zacharieva SZ (2003). Metabolic disturbances in women with polycystic ovary syndrome. Folia Med (Plovdiv) 45, 12–20. - PubMed
-
- Drummond-Barbosa D, and Spradling AC (2001). Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol 231, 265–278. - PubMed
-
- Yan J, Zhou B, Yang J, Tai P, Chen X, Zhang H, Zhang M, and Xia G (2008). Glucose can reverse the effects of acute fasting on mouse ovulation and oocyte maturation. Reprod Fertil Dev 20, 703–712. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

