Telomerase Is Essential for Zebrafish Heart Regeneration
- PMID: 26321646
- PMCID: PMC4589159
- DOI: 10.1016/j.celrep.2015.07.064
Telomerase Is Essential for Zebrafish Heart Regeneration
Abstract
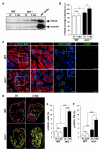
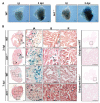
After myocardial infarction in humans, lost cardiomyocytes are replaced by an irreversible fibrotic scar. In contrast, zebrafish hearts efficiently regenerate after injury. Complete regeneration of the zebrafish heart is driven by the strong proliferation response of its cardiomyocytes to injury. Here we show that, after cardiac injury in zebrafish, telomerase becomes hyperactivated, and telomeres elongate transiently, preceding a peak of cardiomyocyte proliferation and full organ recovery. Using a telomerase-mutant zebrafish model, we found that telomerase loss drastically decreases cardiomyocyte proliferation and fibrotic tissue regression after cryoinjury and that cardiac function does not recover. The impaired cardiomyocyte proliferation response is accompanied by the absence of cardiomyocytes with long telomeres and an increased proportion of cardiomyocytes showing DNA damage and senescence characteristics. These findings demonstrate the importance of telomerase function in heart regeneration and highlight the potential of telomerase therapy as a means of stimulating cell proliferation upon myocardial infarction.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
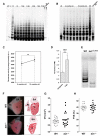
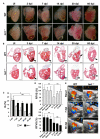
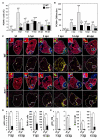

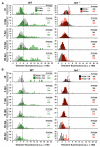
References
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995;57:289–300.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

