Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity
- PMID: 26607794
- PMCID: PMC4671824
- DOI: 10.1016/j.cell.2015.11.009
Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity
Abstract
Extensive cellular heterogeneity exists within specific immune-cell subtypes classified as a single lineage, but its molecular underpinnings are rarely characterized at a genomic scale. Here, we use single-cell RNA-seq to investigate the molecular mechanisms governing heterogeneity and pathogenicity of Th17 cells isolated from the central nervous system (CNS) and lymph nodes (LN) at the peak of autoimmune encephalomyelitis (EAE) or differentiated in vitro under either pathogenic or non-pathogenic polarization conditions. Computational analysis relates a spectrum of cellular states in vivo to in-vitro-differentiated Th17 cells and unveils genes governing pathogenicity and disease susceptibility. Using knockout mice, we validate four new genes: Gpr65, Plzp, Toso, and Cd5l (in a companion paper). Cellular heterogeneity thus informs Th17 function in autoimmunity and can identify targets for selective suppression of pathogenic Th17 cells while potentially sparing non-pathogenic tissue-protective ones.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
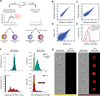

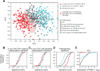


Comment in
-
A Metabolic Switch for Th17 Pathogenicity.Cell. 2015 Dec 3;163(6):1308-10. doi: 10.1016/j.cell.2015.11.033. Cell. 2015. PMID: 26638065
-
T cells: Seq-ing out the 'bad' guys.Nat Rev Immunol. 2016 Jan;16(1):3. doi: 10.1038/nri.2015.15. Epub 2015 Dec 21. Nat Rev Immunol. 2016. PMID: 26688347 No abstract available.
-
Transcriptomic signatures decode Th17 cell pathogenicity.Cell Mol Immunol. 2016 Sep;13(5):557-9. doi: 10.1038/cmi.2016.22. Epub 2016 May 2. Cell Mol Immunol. 2016. PMID: 27133470 Free PMC article. No abstract available.
References
-
- Aust G, Kamprad M, Lamesch P, Schmucking E. CXCR6 within T-helper (Th) and T-cytotoxic (Tc) type 1 lymphocytes in Graves' disease (GD) European journal of endocrinology / European Federation of Endocrine Societies. 2005;152:635–643. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 NS030843/NS/NINDS NIH HHS/United States
- R00 AI110649/AI/NIAID NIH HHS/United States
- NS030843/NS/NINDS NIH HHS/United States
- AI039671/AI/NIAID NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- F32 HD075541/HD/NICHD NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- P30-CA14051/CA/NCI NIH HHS/United States
- P01 NS076410/NS/NINDS NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- R37 NS030843/NS/NINDS NIH HHS/United States
- P50 HG006193/HG/NHGRI NIH HHS/United States
- 4R00AI110649/AI/NIAID NIH HHS/United States
- AI056299/AI/NIAID NIH HHS/United States
- R29 NS030843/NS/NINDS NIH HHS/United States
- NS076410/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

