Suppressor role of activating transcription factor 2 (ATF2) in skin cancer
- PMID: 18227516
- PMCID: PMC2234203
- DOI: 10.1073/pnas.0706057105
Suppressor role of activating transcription factor 2 (ATF2) in skin cancer
Abstract
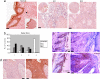
Activating transcription factor 2 (ATF2) regulates transcription in response to stress and growth factor stimuli. Here, we use a mouse model in which ATF2 was selectively deleted in keratinocytes. Crossing the conditionally expressed ATF2 mutant with K14-Cre mice (K14.ATF2(f/f)) resulted in selective expression of mutant ATF2 within the basal layer of the epidermis. When subjected to a two-stage skin carcinogenesis protocol [7,12-dimethylbenz[a]anthracene/phorbol 12-tetradecanoate 13-acetate (DMBA/TPA)], K14.ATF2(f/f) mice showed significant increases in both the incidence and prevalence of papilloma development compared with the WT ATF2 mice. Consistent with these findings, keratinocytes of K14.ATF2(f/f) mice exhibit greater anchorage-independent growth compared with ATF2 WT keratinocytes. Papillomas of K14.ATF2(f/f) mice exhibit reduced expression of presenilin1, which is associated with enhanced beta-catenin and cyclin D1, and reduced Notch1 expression. Significantly, a reduction of nuclear ATF2 and increased beta-catenin expression were seen in samples of squamous and basal cell carcinoma, as opposed to normal skin. Our data reveal that loss of ATF2 transcriptional activity serves to promote skin tumor formation, thereby indicating a suppressor activity of ATF2 in skin tumor formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

