ETV6-NTRK3 fusion oncogene initiates breast cancer from committed mammary progenitors via activation of AP1 complex
- PMID: 18068631
- PMCID: PMC2175032
- DOI: 10.1016/j.ccr.2007.11.012
ETV6-NTRK3 fusion oncogene initiates breast cancer from committed mammary progenitors via activation of AP1 complex
Abstract
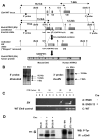
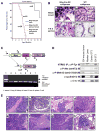
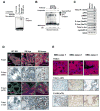
To better understand the cellular origin of breast cancer, we developed a mouse model that recapitulates expression of the ETV6-NTRK3 (EN) fusion oncoprotein, the product of the t(12;15)(p13;q25) translocation characteristic of human secretory breast carcinoma. Activation of EN expression in mammary tissues by Wap-Cre leads to fully penetrant, multifocal malignant breast cancer with short latency. We provide genetic evidence that, in nulliparous Wap-Cre;EN females, committed alveolar bipotent or CD61(+) luminal progenitors are targets of tumorigenesis. Furthermore, EN transforms these otherwise transient progenitors through activation of the AP1 complex. Given the increasing relevance of chromosomal translocations in epithelial cancers, such mice serve as a paradigm for the study of their genetic pathogenesis and cellular origins, and generation of preclinical models.
Figures





References
-
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. - PubMed
-
- Belguise K, Kersual N, Galtier F, Chalbos D. FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene. 2005;24:1434–1444. - PubMed
-
- Booth BW, Boulanger CA, Smith GH. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol. 2007;212:729–736. - PubMed
-
- Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–560. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

