Protein O-GlcNAcylation coupled to Hippo signaling drives vascular dysfunction in diabetic retinopathy
- PMID: 39472558
- PMCID: PMC11522279
- DOI: 10.1038/s41467-024-53601-x
Protein O-GlcNAcylation coupled to Hippo signaling drives vascular dysfunction in diabetic retinopathy
Abstract
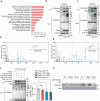
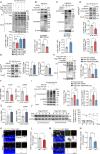
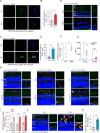
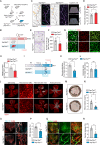
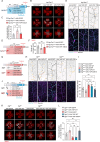
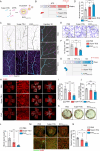
Metabolic disorder significantly contributes to diabetic vascular complications, including diabetic retinopathy, the leading cause of blindness in the working-age population. However, the molecular mechanisms by which disturbed metabolic homeostasis causes vascular dysfunction in diabetic retinopathy remain unclear. O-GlcNAcylation modification acts as a nutrient sensor particularly sensitive to ambient glucose. Here, we observe pronounced O-GlcNAc elevation in retina endothelial cells of diabetic retinopathy patients and mouse models. Endothelial-specific depletion or pharmacological inhibition of O-GlcNAc transferase effectively mitigates vascular dysfunction. Mechanistically, we find that Yes-associated protein (YAP) and Transcriptional co-activator with PDZ-binding motif (TAZ), key effectors of the Hippo pathway, are O-GlcNAcylated in diabetic retinopathy. We identify threonine 383 as an O-GlcNAc site on YAP, which inhibits its phosphorylation at serine 397, leading to its stabilization and activation, thereby promoting vascular dysfunction by inducing a pro-angiogenic and glucose metabolic transcriptional program. This work emphasizes the critical role of the O-GlcNAc-Hippo axis in the pathogenesis of diabetic retinopathy and suggests its potential as a therapeutic target.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ogurtsova, K. et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract.128, 40–50 (2017). - PubMed
-
- Antonetti, D. A., Klein, R. & Gardner, T. W. Diabetic retinopathy. N. Engl. J. Med.366, 1227–1239 (2012). - PubMed
-
- Borrelli, E. et al. Long-term visual outcomes and morphologic biomarkers of vision loss in eyes with diabetic macular edema treated with anti-VEGF Ttherapy. Am. J. Ophthalmol.235, 80–89 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
- 82122018/National Natural Science Foundation of China (National Science Foundation of China)
- 32171101/National Natural Science Foundation of China (National Science Foundation of China)
- 8230041151/National Natural Science Foundation of China (National Science Foundation of China)
- 82020108007/National Natural Science Foundation of China (National Science Foundation of China)
- 23JCJQJC00050/Natural Science Foundation of Tianjin City (Natural Science Foundation of Tianjin)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

