Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice
- PMID: 26483457
- PMCID: PMC4640735
- DOI: 10.1073/pnas.1510595112
Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice
Abstract
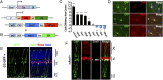
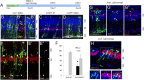
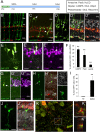
Müller glial cells are the source of retinal regeneration in fish and birds; although this process is efficient in fish, it is less so in birds and very limited in mammals. It has been proposed that factors necessary for providing neurogenic competence to Müller glia in fish and birds after retinal injury are not expressed in mammals. One such factor, the proneural transcription factor Ascl1, is necessary for retinal regeneration in fish but is not expressed after retinal damage in mice. We previously reported that forced expression of Ascl1 in vitro reprograms Müller glia to a neurogenic state. We now test whether forced expression of Ascl1 in mouse Müller glia in vivo stimulates their capacity for retinal regeneration. We find that transgenic expression of Ascl1 in adult Müller glia in undamaged retina does not overtly affect their phenotype; however, when the retina is damaged, the Ascl1-expressing glia initiate a response that resembles the early stages of retinal regeneration in zebrafish. The reaction to injury is even more pronounced in Müller glia in young mice, where the Ascl1-expressing Müller glia give rise to amacrine and bipolar cells and photoreceptors. DNaseI-seq analysis of the retina and Müller glia shows progressive reduction in accessibility of progenitor gene cis-regulatory regions consistent with the reduction in their reprogramming. These results show that at least one of the differences between mammal and fish Müller glia that bears on their difference in regenerative potential is the proneural transcription factor Ascl1.
Keywords: eye; glia; neurogenesis; regeneration; reprogramming.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Karl MO, Reh TA. Studying the generation of regenerated retinal neuron from Müller glia in the mouse eye. Methods Mol Biol. 2012;884:213–227. - PubMed
-
- Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4(3):247–252. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

