Proteolytic activation and function of the cytokine Spätzle in the innate immune response of a lepidopteran insect, Manduca sexta
- PMID: 19968713
- PMCID: PMC2805777
- DOI: 10.1111/j.1742-4658.2009.07465.x
Proteolytic activation and function of the cytokine Spätzle in the innate immune response of a lepidopteran insect, Manduca sexta
Abstract
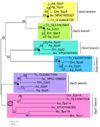
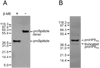
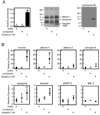
The innate immune response of insects includes induced expression of genes encoding a variety of antimicrobial peptides. The signaling pathways that stimulate this gene expression have been well characterized by genetic analysis in Drosophila melanogaster, but are not well understood in most other insect species. One such pathway involves proteolytic activation of a cytokine called Spätzle, which functions in dorsal-ventral patterning in early embryonic development and in the antimicrobial immune response in larvae and adults. We have investigated the function of Spätzle in a lepidopteran insect, Manduca sexta, in which hemolymph proteinases activated during immune responses have been characterized biochemically. Two cDNA isoforms for M. sexta Spätzle-1 differ because of alternative splicing, resulting in a 10 amino acid residue insertion in the pro-region of proSpätzle-1B that is not present in proSpätzle-1A. The proSpätzle-1A cDNA encodes a 32.7 kDa polypeptide that is 23% and 44% identical to D. melanogaster and Bombyx mori Spätzle-1, respectively. Recombinant proSpätzle-1A was a disulfide-linked homodimer. M. sexta hemolymph proteinase 8 cleaved proSpätzle-1A to release Spätzle-C108, a dimer of the C-terminal 108 residue cystine-knot domain. Injection of Spätzle-C108, but not proSpätzle-1A, into larvae stimulated expression of several antimicrobial peptides and proteins, including attacin-1, cecropin-6, moricin, lysozyme, and the immunoglobulin domain protein hemolin, but did not significantly affect the expression of two bacteria-inducible pattern recognition proteins, immulectin-2 and beta-1,3-glucan recognition protein-2. The results of this and other recent studies support a model for a pathway in which the clip-domain proteinase pro-hemolymph proteinase 6 becomes activated in plasma upon exposure to Gram-negative or Gram-positive bacteria or to beta-1,3-glucan. Hemolymph proteinase 6 then activates pro-hemolymph proteinase 8, which in turn activates Spätzle-1. The resulting Spätzle-C108 dimer is likely to function as a ligand to activate a Toll pathway in M. sexta as a response to a wide variety of microbial challenges, stimulating a broad response to infection. Structured digital abstract * MINT-7295125: Spätzle 1A (uniprotkb:C8BMD1) and Spätzle 1A (uniprotkb:C8BMD1) bind (MI:0407) by comigration in gel electrophoresis (MI:0807).
Figures





References
-
- Kanost MR, Gorman MJ. Phenoloxidase in insect immunity. In: Beckage N, editor. Insect Immunology. San Diego: Academic Press/Elsevier; 2008. pp. 69–96.
-
- Cerenius L, Lee BL, Soderhall K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29:263–271. - PubMed
-
- Yu X, Prakash O, Kanost MR. Insect ENF family peptides with diverse biological activities display similar core structures in solution. Medical Chem. Res. 2001;10:493–501.
-
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat.Rev.Immunol. 2007;7:862–874. - PubMed
-
- Gillespie JP, Kanost MR, Trenczek T. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997;42:611–643. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

