Genomic analysis of oceanic cyanobacterial myoviruses compared with T4-like myoviruses from diverse hosts and environments
- PMID: 20662890
- PMCID: PMC3037559
- DOI: 10.1111/j.1462-2920.2010.02280.x
Genomic analysis of oceanic cyanobacterial myoviruses compared with T4-like myoviruses from diverse hosts and environments
Abstract
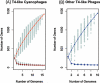
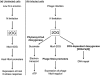
T4-like myoviruses are ubiquitous, and their genes are among the most abundant documented in ocean systems. Here we compare 26 T4-like genomes, including 10 from non-cyanobacterial myoviruses, and 16 from marine cyanobacterial myoviruses (cyanophages) isolated on diverse Prochlorococcus or Synechococcus hosts. A core genome of 38 virion construction and DNA replication genes was observed in all 26 genomes, with 32 and 25 additional genes shared among the non-cyanophage and cyanophage subsets, respectively. These hierarchical cores are highly syntenic across the genomes, and sampled to saturation. The 25 cyanophage core genes include six previously described genes with putative functions (psbA, mazG, phoH, hsp20, hli03, cobS), a hypothetical protein with a potential phytanoyl-CoA dioxygenase domain, two virion structural genes, and 16 hypothetical genes. Beyond previously described cyanophage-encoded photosynthesis and phosphate stress genes, we observed core genes that may play a role in nitrogen metabolism during infection through modulation of 2-oxoglutarate. Patterns among non-core genes that may drive niche diversification revealed that phosphorus-related gene content reflects source waters rather than host strain used for isolation, and that carbon metabolism genes appear associated with putative mobile elements. As well, phages isolated on Synechococcus had higher genome-wide %G+C and often contained different gene subsets (e.g. petE, zwf, gnd, prnA, cpeT) than those isolated on Prochlorococcus. However, no clear diagnostic genes emerged to distinguish these phage groups, suggesting blurred boundaries possibly due to cross-infection. Finally, genome-wide comparisons of both diverse and closely related, co-isolated genomes provide a locus-to-locus variability metric that will prove valuable for interpreting metagenomic data sets.
© 2010 Society for Applied Microbiology and Blackwell Publishing Ltd.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

