Genome-wide analyses reveal a role for peptide hormones in planarian germline development
- PMID: 20967238
- PMCID: PMC2953531
- DOI: 10.1371/journal.pbio.1000509
Genome-wide analyses reveal a role for peptide hormones in planarian germline development
Erratum in
-
Correction: Genome-Wide Analyses Reveal a Role for Peptide Hormones in Planarian Germline Development.PLoS Biol. 2015 Aug 14;13(8):e1002234. doi: 10.1371/journal.pbio.1002234. eCollection 2015 Aug. PLoS Biol. 2015. PMID: 26275013 Free PMC article. No abstract available.
Abstract
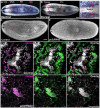
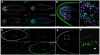
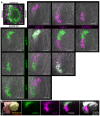
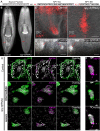
Bioactive peptides (i.e., neuropeptides or peptide hormones) represent the largest class of cell-cell signaling molecules in metazoans and are potent regulators of neural and physiological function. In vertebrates, peptide hormones play an integral role in endocrine signaling between the brain and the gonads that controls reproductive development, yet few of these molecules have been shown to influence reproductive development in invertebrates. Here, we define a role for peptide hormones in controlling reproductive physiology of the model flatworm, the planarian Schmidtea mediterranea. Based on our observation that defective neuropeptide processing results in defects in reproductive system development, we employed peptidomic and functional genomic approaches to characterize the planarian peptide hormone complement, identifying 51 prohormone genes and validating 142 peptides biochemically. Comprehensive in situ hybridization analyses of prohormone gene expression revealed the unanticipated complexity of the flatworm nervous system and identified a prohormone specifically expressed in the nervous system of sexually reproducing planarians. We show that this member of the neuropeptide Y superfamily is required for the maintenance of mature reproductive organs and differentiated germ cells in the testes. Additionally, comparative analyses of our biochemically validated prohormones with the genomes of the parasitic flatworms Schistosoma mansoni and Schistosoma japonicum identified new schistosome prohormones and validated half of all predicted peptide-encoding genes in these parasites. These studies describe the peptide hormone complement of a flatworm on a genome-wide scale and reveal a previously uncharacterized role for peptide hormones in flatworm reproduction. Furthermore, they suggest new opportunities for using planarians as free-living models for understanding the reproductive biology of flatworm parasites.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Littlewood D. T. J, Bray R. A. In: Interrelationships of the platyhelminthes. Warren A, editor. London: Taylor & Francis; 2001. 356
-
- Hyman L. The invertebrates: platyhelminthes and rhynchocoela the acoelomate bilateria. New York: McGraw-Hill Book Company, Inc.; 1951. 550
-
- Curtis W. C. The life history, the normal fission and the reproductive organs of Planaria maculata. Proceedings of the Boston Society of Natural History. 1902;30:515–559.
-
- Severinghaus A. E. Memoirs: sex studies on Schistosoma japonicum. Quarterly Journal of Microscopical Science. 1928;71:653–702.
-
- Erasmus D. A. A comparative study of the reproductive system of mature, immature and “unisexual” female Schistosoma mansoni. Parasitology. 1973;67:165–183. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

