Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study
- PMID: 28522352
- PMCID: PMC5828518
- DOI: 10.1016/S2213-2600(17)30174-1
Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study
Erratum in
-
Corrections.Lancet Respir Med. 2017 Jul;5(7):e26. doi: 10.1016/S2213-2600(17)30225-4. Lancet Respir Med. 2017. PMID: 28664867 Free PMC article. No abstract available.
Abstract
Background: Influenza causes substantial morbidity and mortality despite available treatments. Anecdotal reports suggest that plasma with high antibody titres to influenza might be of benefit in the treatment of severe influenza.
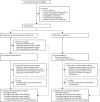
Methods: In this randomised, open-label, multicentre, phase 2 trial, 29 academic medical centres in the USA assessed the safety and efficacy of anti-influenza plasma with haemagglutination inhibition antibody titres of 1:80 or more to the infecting strain. Hospitalised children and adults (including pregnant women) with severe influenza A or B (defined as the presence of hypoxia or tachypnoea) were randomly assigned to receive either two units (or paediatric equivalent) of anti-influenza plasma plus standard care, versus standard care alone, and were followed up for 28 days. The primary endpoint was time to normalisation of patients' respiratory status (respiratory rate of ≤20 breaths per min for adults or age-defined thresholds of 20-38 breaths per min for children) and a room air oxygen saturation of 93% or more. This study is registered with ClinicalTrials.gov, number NCT01052480.
Findings: Between Jan 13, 2011, and March 2, 2015, 113 participants were screened for eligibility and 98 were randomly assigned from 20 out of 29 participating sites. Of the participants with confirmed influenza (by PCR), 28 (67%) of 42 in the plasma plus standard care group normalised their respiratory status by day 28 compared with 24 (53%) of 45 participants on standard care alone (p=0·069). The hazard ratio (HR) comparing plasma plus standard care with standard care alone was 1·71 (95% CI 0·96-3·06). Six participants died, one (2%) from the plasma plus standard care group and five (10%) from the standard care group (HR 0·19 [95% CI 0·02-1·65], p=0·093). Participants in the plasma plus standard care group had non-significant reductions in days in hospital (median 6 days [IQR 4-16] vs 11 days [5-25], p=0·13) and days on mechanical ventilation (median 0 days [IQR 0-6] vs 3 days [0-14], p=0·14). Fewer plasma plus standard care participants had serious adverse events compared with standard care alone recipients (nine [20%] of 46 vs 20 [38%] of 52, p=0·041), the most frequent of which were acute respiratory distress syndrome (one [2%] vs two [4%] patients) and stroke (one [2%] vs two [4%] patients).
Interpretation: Although there was no significant effect of plasma treatment on the primary endpoint, the treatment seemed safe and well tolerated. A phase 3 randomised trial is now underway to further assess this intervention.
Funding: National Institute of Allergy and Infectious Diseases, US National Institutes of Health.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Serotherapy for patients with severe influenza.Lancet Respir Med. 2017 Jun;5(6):462-464. doi: 10.1016/S2213-2600(17)30173-X. Epub 2017 May 15. Lancet Respir Med. 2017. PMID: 28522353 No abstract available.
-
Potential and challenges of serotherapy for severe influenza.Lancet Respir Med. 2017 Aug;5(8):e27. doi: 10.1016/S2213-2600(17)30266-7. Lancet Respir Med. 2017. PMID: 28748808 Free PMC article. No abstract available.
References
-
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. - PubMed
-
- The ANZIC Influenza Investigators Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. - PubMed
-
- Davies A, Jones D, Bailey M, Australia and New Zeland Extracorporeal Membrane Oxygenation Influenza Investigators Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. - PubMed
-
- Dominguez-Cherit G, Lapinsky SE, Macias AE. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

