HIV drug resistance (HIVDR) in antiretroviral therapy-naïve patients in Tanzania not eligible for WHO threshold HIVDR survey is dramatically high
- PMID: 21886779
- PMCID: PMC3158766
- DOI: 10.1371/journal.pone.0023091
HIV drug resistance (HIVDR) in antiretroviral therapy-naïve patients in Tanzania not eligible for WHO threshold HIVDR survey is dramatically high
Abstract
Background: The World Health Organization (WHO) has recommended guidelines for a HIV drug resistance (HIVDR) survey for resource-limited countries. Eligibility criteria for patients include age below 25 years in order to focus on the prevalence of transmitted HIVDR (tHIVDR) in newly-infected individuals. Most of the participating sites across Africa have so far reported tHIVDR prevalences of below 5%. In this study we investigated whether the rate of HIVDR in patients <25 years is representative for HIVDR in the rest of the therapy-naïve population.
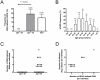
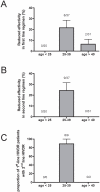
Methods and findings: HIVDR was determined in 88 sequentially enrolled ART-naïve patients from Mwanza, Tanzania (mean age 35.4 years). Twenty patients were aged <25 years and 68 patients were aged 25-63 years. The frequency of HIVDR in the study population was 14.8% (95%; CI 0.072-0.223) and independent of NVP-resistance induced by prevention of mother-to-child transmission programs. Patients >25 years had a significantly higher HIVDR frequency than younger patients (19.1%; 95% CI 0.095-0.28) versus 0%, P = 0.0344). In 2 out of the 16 patients with HIVDR we found traces of antiretrovirals (ARVs) in plasma.
Conclusions: ART-naïve patients aged over 25 years exhibited significantly higher HIVDR than younger patients. Detection of traces of ARVs in individuals with HIVDR suggests that besides transmission, undisclosed misuse of ARVs may constitute a significant factor in the generation of the observed high HIVDR rate. The current WHO tHIVDR survey that is solely focused on the transmission of HIVDR and that excludes patients over 25 years of age may therefore result in substantial underestimation of the prevalence of HIVDR in the therapy-naïve population. Similar studies should be performed also in other areas to test whether the so far reported optimistic picture of low HIVDR prevalence in young individuals is really representative for the rest of the ART-naïve HIV-infected population.
Conflict of interest statement
Figures
References
-
- Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. - PubMed
-
- WHO progress report. 2010. http://www.who.int/hiv/pub/2010progressreport/report/en/index.html. Accessed 2011 May 15th.
-
- National Guidelines for the management of HIV and AIDS in Tanzania.
-
- Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization's global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(Suppl 2):1–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

