PB2 and hemagglutinin mutations are major determinants of host range and virulence in mouse-adapted influenza A virus
- PMID: 20702632
- PMCID: PMC2950562
- DOI: 10.1128/JVI.01187-10
PB2 and hemagglutinin mutations are major determinants of host range and virulence in mouse-adapted influenza A virus
Abstract
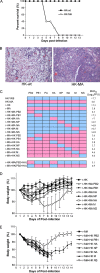
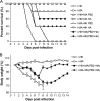
Serial mouse lung passage of a human influenza A virus, A/Hong Kong/1/68 (H3N2) (HK-wt), produced a mouse-adapted variant, MA, with nine mutations that was >10(3.8)-fold more virulent. In this study, we demonstrate that MA mutations of the PB2 (D701N) and hemagglutinin (HA) (G218W in HA1 and T156N in HA2) genes were the most adaptive genetic determinants for increased growth and virulence in the mouse model. Recombinant viruses expressing each of the mutated MA genome segments on the HK-wt backbone showed significantly increased disease severity, whereas only the mouse-adapted PB2 gene increased virulence, as determined by the 50% lethal dose ([LD(50)] >10(1.4)-fold). The converse comparisons of recombinant MA viruses expressing each of the HK-wt genome segments showed the greatest decrease in virulence due to the HA gene (10(2)-fold), with lesser decreases due to the M1, NS1, NA, and PB1 genes (10(0.3)- to 10(0.8)-fold), and undetectable effects on the LD(50) for the PB2 and NP genes. The HK PB2 gene did, however, attenuate MA infection, as measured by weight loss and time to death. Replication of adaptive mutations in vivo and in vitro showed both viral gene backbone and host range effects. Minigenome transcription assays showed that PB1 and PB2 mutations increased polymerase activity and that the PB2 D701N mutation was comparable in effect to the mammalian adaptive PB2 E627K mutation. Our results demonstrate that host range and virulence are controlled by multiple genes, with major roles for mutations in PB2 and HA.
Figures



References
-
- Brown, E. G., and J. E. Bailly. 1999. Genetic analysis of mouse-adapted influenza A virus identifies roles for the NA, PB1, and PB2 genes in virulence. Virus Res. 61:63-76. - PubMed
-
- de Jong, M. D., C. P. Simmons, T. T. Thanh, V. M. Hien, G. J. Smith, T. N. Chau, D. M. Hoang, N. V. Chau, T. H. Khanh, V. C. Dong, P. T. Qui, B. V. Cam, Q. Ha do, Y. Guan, J. S. Peiris, N. T. Chinh, T. T. Hien, and J. Farrar. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12:1203-1207. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

