Combination treatment of fingolimod with antidepressants in relapsing-remitting multiple sclerosis patients with depression: a multicentre, open-label study - REGAIN
- PMID: 27582893
- PMCID: PMC4994781
- DOI: 10.1177/1756285616651197
Combination treatment of fingolimod with antidepressants in relapsing-remitting multiple sclerosis patients with depression: a multicentre, open-label study - REGAIN
Abstract
Objectives: Approximately one in two patients with multiple sclerosis (MS) suffer from comorbid depression. The primary objective of this study was to evaluate the safety and tolerability of fingolimod and antidepressant combination in relapsing-remitting MS patients with mild-to-moderate depression. Efficacy outcome variables were quality of life (QoL), fatigue, disability and depression.
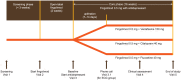
Methods: Patients received open-label fingolimod 0.5 mg over 2 weeks, followed by fingolimod plus citalopram (40 mg), fluoxetine (40 mg) or venlafaxine (150 mg) over 16 weeks. The antidepressant was selected at the physician's discretion.
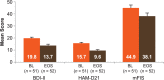
Results: In total, 54 patients were recruited at 25 centres across Germany. No new safety signals (including cardiac) emerged compared with previous clinical studies. Adverse events (mostly mild-to-moderate) were reported in 43 patients. A total of three patients had serious adverse events and 10 discontinued the study. QoL (mean [95% confidence interval]) improved by 2.2 (-3.3, -1.2; Patient Reported Indices for MS questionnaire), fatigue by 8.2 (-13.1, -3.3; modified Fatigue Impact Scale) and depression by 6.3 (-8.4, -4.2; Hamilton Depression Scale) points. However, the results must be interpreted cautiously owing to limited patient numbers.
Conclusions: Combination of fingolimod with antidepressant medication showed no unexpected safety signals. Patient-reported outcomes (QoL, disability, fatigue and depression) remained stable or improved.
Keywords: FTY720; Gilenya®; depression; fatigue; fingolimod; multiple sclerosis.
Conflict of interest statement
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Antonios Bayas received honoraria for consulting and/or as a speaker from Merck Serono, Germany; Biogen, Germany, Poland; Bayer Vital, Germany; Novartis, Germany, Austria; Sanofi/Genzyme, Germany, Netherlands; Roche, Germany and TEVA, Germany, for trial activities from Merck Serono, Germany; Biogen, Germany and Novartis, Germany and grants for congress trips and participation from Novartis, Germany; Biogen, Germany; Sanofi/Genzyme, Germany; and Merck Serono, Germany. Katrin Schuh, Monika Baier and Stefan Viktor Vormfelde are employees of Novartis Pharma GmbH, Nuremberg, Germany.
Figures

References
-
- Beach S., Kostis W., Celano C., Januzzi J., Ruskin J., Noseworthy P., et al. (2014) Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J Clin Psychiatry 75: e441–e449. - PubMed
-
- Beck A., Steer R., Ball R., Ranieri W. (1996) Comparison of Beck Depression Inventories -Ia and -II in psychiatric outpatients. J Pers Assess 67: 588–597. - PubMed
-
- Beiske A., Svensson E., Sandanger I., Czujko B., Pedersen E., Aarseth J., et al. (2008) Depression and anxiety amongst multiple sclerosis patients. Eur J Neurol 15: 239–245. - PubMed
-
- Benedetti F., Campori E., Colombo C., Smeraldi E. (2004) Fluvoxamine treatment of major depression associated with multiple sclerosis. J Neuropsychiatry Clin Neurosci 16: 364–366. - PubMed
-
- Bermel R., Hashmonay R., Meng X., Randhawa S., Von Rosenstiel P., Sfikas N., et al. (2015) Fingolimod first-dose effects in patients with relapsing multiple sclerosis concomitantly receiving selective serotonin-reuptake inhibitors. Mult Scler Relat Disord 4: 273–280. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

