Analysis of human factor H-related gene and protein expressed in rheumatoid arthritis synovium identifies a novel mechanism promoting dysregulated complement pathway activation
- PMID: 40467630
- PMCID: PMC12137822
- DOI: 10.1038/s41598-025-03589-1
Analysis of human factor H-related gene and protein expressed in rheumatoid arthritis synovium identifies a novel mechanism promoting dysregulated complement pathway activation
Abstract
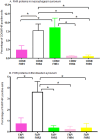
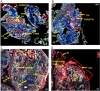
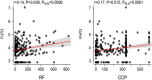
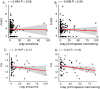
Factor H (FH) is a negative regulator of the alternative pathway (AP) of complement however, five human factor H-related (FHR) proteins, can also function ex vivo as positive regulators. We compare bulk FH and FHR mRNA expressions in both the human rheumatoid arthritis (RA) synovium and blood cells from the Pathobiology of Early Arthritis Cohort (PEAC) and the Stratification of biological therapies for Rheumatoid Arthritis by Pathobiology (STRAP) Cohort. FH and FHR proteins were detected using multiplexed immunohistochemistry (MIHC) in synovium. In three pathotypes, in the synovium, no differences were found in the expression of FHR mRNA. In the synovium, a significant negative correlation was observed between FH expression and the disease activity score and X-ray joint space narrowing. In RA patients, there was a significant positive correlation between FHR3 mRNA level, anti-cyclic citrullinated peptide (CCP) antibodies and rheumatoid factor (RF). FHR proteins were co-localized in the synovial lining area along with complement C3 while FH was almost undetectable in the synovial lining but abundant in sub-synovial lining areas. We do not know whether FH and FHR proteins are locally generated and deposited in synovium or come from circulation. In sum, due to the absence of FH but the presence of FHRs, the synovial lining might fail to be protected from complement-mediated attack, and FHR3 may play a particularly important pathogenic role.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. NKB: royalties, and patents for the treatment of inflammatory diseases using anti-C5aR1ab-C5siRNA conjugate. LWM: none; KDD: none; DS, none; RIS: none; RMF: none; JS, none; RL: none; CP: none; MJL: none; VMH: royalties, consulting income, stock, and stock options in complement therapeutics companies. Ethical approval and consent to participate: Written ethical informed consent was obtained from all eRA patients according to the preapproved Colorado Multiple Institutional Review Board protocol (COMIRB # 20-1908 and 15-1389). All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols related to these studies were approved by the Institutional Review Board committee.
Figures




References
-
- Cross, M. et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann. Rheumatol. Dis.73, 1316–1322 (2014). - PubMed
-
- Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers. 4, 18001 (2018). - PubMed
-
- Prevoo, M. L., van ‘t Hof, M. A., Kuper, H. H., van Leeuwen, M. A. & van de Putte, L. B. and P. L. van Riel. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheumatol 38: 44–48. (1995). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

