Study protocol for a multicentre, 2×2 factorial, randomised, controlled trial evaluating the interest of intravenous iron and tranexamic acid to reduce blood transfusion in hip fracture patients (the HiFIT study)
- PMID: 33455926
- PMCID: PMC7813351
- DOI: 10.1136/bmjopen-2020-040273
Study protocol for a multicentre, 2×2 factorial, randomised, controlled trial evaluating the interest of intravenous iron and tranexamic acid to reduce blood transfusion in hip fracture patients (the HiFIT study)
Abstract
Introduction: Blood transfusion and anaemia are frequent and are associated with poor outcomes in patients with hip fracture (HF). We hypothesised that preoperative intravenous iron and tranexamic acid (TXA) may reduce the transfusion rate in these patients.
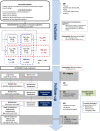
Methods and analysis: The HiFIT study is a multicentre, 2×2 factorial, randomised, double-blinded, controlled trial evaluating the effect of iron isomaltoside (IIM) (20 mg/kg) vs placebo and of TXA (intravenously at inclusion and topically during surgery) versus placebo on transfusion rate during hospitalisation, in patients undergoing emergency surgery for HF and having a preoperative haemoglobin between 95 and 130 g/L. 780 patients are expected. The primary endpoint is the proportion of patients receiving an allogenic blood transfusion of packed red blood cells from the day of surgery until hospital discharge (or until D30 if patient is still hospitalised). Enrolment started on March 2017 in 11 French hospitals. The study was stopped between July 2017 and August 2018 (because of investigation of serious AEs with IIM in Spain) and slowed down since March 2020 (COVID-19 crisis). The expected date of final follow-up is May 2022. Analyses of the intent-to-treat and per-protocol populations are planned.
Ethics and dissemination: The HiFIT trial protocol has been approved by the Ethics Committee of Comité de Protection des Personnes Ouest II and the French authorities (ANSM). It will be carried out according to the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines. The results will be disseminated through presentation at scientific conferences and publication in peer-reviewed journals. The HiFIT trial will be the largest study evaluating iron and TXA in patients with HF.
Trial registration number: clinicalTrials.gov identifier: NCT02972294; EudraCT Number 2016-003087-40.
Keywords: anaemia; bleeding disorders & coagulopathies; blood bank & transfusion medicine; hip.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SL: Lecturer personally funded by Pfizer, Vifor Pharma, Masimo; consulting for Pfizer, Vifor Pharma; research support from Vifor Pharma.
Figures
References
-
- Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States. numbers, costs, and potential effects of postmenopausal estrogen. Clin Orthop Relat Res 1990;252:163–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
