Colorectal carcinoma: the management of polyps, (neo)adjuvant therapy, and the treatment of metastases
- PMID: 20062582
- PMCID: PMC2803611
- DOI: 10.3238/arztebl.2009.0843
Colorectal carcinoma: the management of polyps, (neo)adjuvant therapy, and the treatment of metastases
Erratum in
- Dtsch Arztebl Int. 2010 Feb;107(5):74
Abstract
Background: Colorectal carcinoma (CRC) is the second most common type of cancer in Germany. In view of recent major changes in the diagnosis and treatment of CRC, the S3 guideline for CRC published in its full version in 2004 was partially updated in 2008 and again in 2009.
Method: The literature was systematically searched for all articles published from 2004 onward concerning polyp management, (neo-)adjuvant treatment, and treatment of metastatic disease. Evidence-based recommendations were developed in a consensus conference.
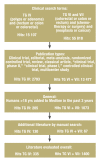
Results: For some patients who have undergone polypectomy, the time to follow-up with colonoscopy can be lengthened. In UICC stage III colon cancer, adjuvant chemotherapy with an oxaliplatin-based regimen is recommended. In stage II colon cancer, adjuvant chemotherapy should be considered mainly when risk factors are present. In stages II and III, neo-adjuvant therapy should be given before resection in rectal cancer. In patients with metastatic disease, the use of all possible treatment options results in a median overall survival time of 24 months. In some patients with primarily non-resectable liver metastases, systemic treatment may enable a secondary, potentially curative resection. Therapeutic agents are chosen individually on the basis of clinical factors including the goal of treatment, the patient's general condition, and tumor molecular markers.
Conclusion: The S3 guideline contains evidence-based recommendations for the diagnosis and treatment of colorectal carcinoma. Broad implementation of the guideline will be essential for improved patient care.
Keywords: adjuvant therapy; cancer treatment; colorectal carcinoma; palliative treatment; polyps.
Figures
Comment in
-
Resistance tests were not mentioned.Dtsch Arztebl Int. 2010 Jun;107(22):399; author reply 399-400. doi: 10.3238/arztebl.2010.0399a. Epub 2010 Jun 4. Dtsch Arztebl Int. 2010. PMID: 20574557 Free PMC article. No abstract available.
References
-
- Robert Koch-Institut. Krebs in Deutschland. www.rki.de. 2008
-
- Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. - PubMed
-
- Schmiegel W, Reinacher-Schick A, Arnold D, et al. S3-Leitlinie „Kolorektales Karzinom“ - Aktualisierung 2008. Z Gastroenterol. 2008;46:799–840. - PubMed
-
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical