Prophylactic catheter ablation for the prevention of defibrillator therapy
- PMID: 18160685
- PMCID: PMC2390777
- DOI: 10.1056/NEJMoa065457
Prophylactic catheter ablation for the prevention of defibrillator therapy
Abstract
Background: For patients who have a ventricular tachyarrhythmic event, implantable cardioverter-defibrillators (ICDs) are a mainstay of therapy to prevent sudden death. However, ICD shocks are painful, can result in clinical depression, and do not offer complete protection against death from arrhythmia. We designed this randomized trial to examine whether prophylactic radiofrequency catheter ablation of arrhythmogenic ventricular tissue would reduce the incidence of ICD therapy.
Methods: Eligible patients with a history of a myocardial infarction underwent defibrillator implantation for spontaneous ventricular tachycardia or fibrillation. The patients did not receive antiarrhythmic drugs. Patients were randomly assigned to defibrillator implantation alone or defibrillator implantation with adjunctive catheter ablation (64 patients in each group). Ablation was performed with the use of a substrate-based approach in which the myocardial scar is mapped and ablated while the heart remains predominantly in sinus rhythm. The primary end point was survival free from any appropriate ICD therapy.
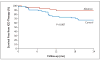
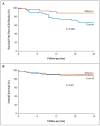
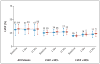
Results: The mortality rate 30 days after ablation was zero, and there were no significant changes in ventricular function or functional class during the mean (+/-SD) follow-up period of 22.5+/-5.5 months. Twenty-one patients assigned to defibrillator implantation alone (33%) and eight patients assigned to defibrillator implantation plus ablation (12%) received appropriate ICD therapy (antitachycardia pacing or shocks) (hazard ratio in the ablation group, 0.35; 95% confidence interval, 0.15 to 0.78, P=0.007). Among these patients, 20 in the control group (31%) and 6 in the ablation group (9%) received shocks (P=0.003). Mortality was not increased in the group assigned to ablation as compared with the control group (9% vs. 17%, P=0.29).
Conclusions: In this randomized trial, prophylactic substrate-based catheter ablation reduced the incidence of ICD therapy in patients with a history of myocardial infarction who received ICDs for the secondary prevention of sudden death. (Current Controlled Trials number, ISRCTN62488166 [controlled-trials.com].).
Copyright 2007 Massachusetts Medical Society.
Figures

Comment in
-
Ablation after ICD implantation--bridging the gap between promise and practice.N Engl J Med. 2007 Dec 27;357(26):2717-9. doi: 10.1056/NEJMe0706860. N Engl J Med. 2007. PMID: 18160692 No abstract available.
-
Catheter ablation in patients with ICDs.N Engl J Med. 2008 May 22;358(21):2295. doi: 10.1056/NEJMc080125. N Engl J Med. 2008. PMID: 18499579 No abstract available.
-
Catheter ablation in patients with ICDs.N Engl J Med. 2008 May 22;358(21):2295-6. N Engl J Med. 2008. PMID: 18504829 No abstract available.
References
-
- Goldberger Z, Lampert R. Implantable cardioverter-defibrillators: expanding indications and technologies. JAMA. 2006;295:809–18. - PubMed
-
- Kamphuis HCM, de Leeuw RJ, Derksen R, Hauer RN, Winnubst JA. Implantable cardioverter defibrillator recipients: quality of life in recipients with and without ICD shock delivery: a prospective study. Europace. 2003;5:381–9. - PubMed
-
- Bilge AK, Ozben B, Demircan S, Cinar M, Yilmaz E, Adalet K. Depression and anxiety status of patients with implantable cardioverter defibrillator and precipitating factors. Pacing Clin Electrophysiol. 2006;29:619–26. - PubMed
-
- Villacastín J, Almendral J, Arenal A, et al. Incidence and clinical significance of multiple consecutive, appropriate, high-energy discharges in patients with implanted cardioverter-defibrillators. Circulation. 1996;93:753–62. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
