Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria
- PMID: 21832085
- PMCID: PMC3186357
- DOI: 10.1074/jbc.M111.248138
Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria
Abstract
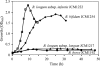
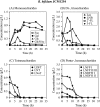
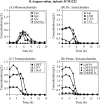
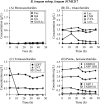
The bifidogenic effect of human milk oligosaccharides (HMOs) has long been known, yet the precise mechanism underlying it remains unresolved. Recent studies show that some species/subspecies of Bifidobacterium are equipped with genetic and enzymatic sets dedicated to the utilization of HMOs, and consequently they can grow on HMOs; however, the ability to metabolize HMOs has not been directly linked to the actual metabolic behavior of the bacteria. In this report, we clarify the fate of each HMO during cultivation of infant gut-associated bifidobacteria. Bifidobacterium bifidum JCM1254, Bifidobacterium longum subsp. infantis JCM1222, Bifidobacterium longum subsp. longum JCM1217, and Bifidobacterium breve JCM1192 were selected for this purpose and were grown on HMO media containing a main neutral oligosaccharide fraction. The mono- and oligosaccharides in the spent media were labeled with 2-anthranilic acid, and their concentrations were determined at various incubation times using normal phase high performance liquid chromatography. The results reflect the metabolic abilities of the respective bifidobacteria. B. bifidum used secretory glycosidases to degrade HMOs, whereas B. longum subsp. infantis assimilated all HMOs by incorporating them in their intact forms. B. longum subsp. longum and B. breve consumed lacto-N-tetraose only. Interestingly, B. bifidum left degraded HMO metabolites outside of the cell even when the cells initiate vegetative growth, which indicates that the different species/subspecies can share the produced sugars. The predominance of type 1 chains in HMOs and the preferential use of type 1 HMO by infant gut-associated bifidobacteria suggest the coevolution of the bacteria with humans.
Figures


References
-
- Newburg D. S., Neubauer S. H. (1995) in Handbook of Milk Composition (Jensen R G. ed) pp. 273–349, Academic Press, San Diego
-
- Ninonuevo M. R., Park Y., Yin H., Zhang J., Ward R. E., Clowers B. H., German J. B., Freeman S. L., Killeen K., Grimm R., Lebrilla C. B. (2006) J. Agric. Food Chem. 54, 7471–7480 - PubMed
-
- Urashima T., Kitaoka M., Terabayashi T., Fukuda K., Ohnishi M., Kobata A. (2011) in Oligosaccharides: Sources, Properties, and Applications (Gordon N. G. ed) pp. 1–58, Nova Science Publishers, New York
-
- Amano J., Osanai M., Orita T., Sugahara D., Osumi K. (2009) Glycobiology 19, 601–614 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

